Read up on the latest publications in diversity-oriented synthesis, rational drug design, chemical biology, and drug discovery research from the Tan Lab!
Selected Publications: Text Only | Graphical Abstracts
Complete Publications: Text Only | Graphical Abstracts
Full-text versions of articles are available from the publisher with online journal subscription. PubMed Central (PMC) versions of articles are available free of charge.
[ 2018 | 2017 | 2016 | 2015 | 2014 | 2013 | 2012 | 2011 | 2010 | 2009 | 2008 | 2007 | 2006 | 2005 | 2004 | 2003 | 2002 | Postdoctoral | Graduate | Undergraduate ]
Tan Lab Publications
Defining new chemical space for drug penetration into Gram-negative bacteria.
Zhao, S.; Adamiak, J. W.; Bonifay, V.; Mehla, J.; Zgurskaya, H. I.; Tan, D. S.* Nat Chem Biol 2020, 16, 1293–1302.
[ Abstract | PubMed | PMC ]
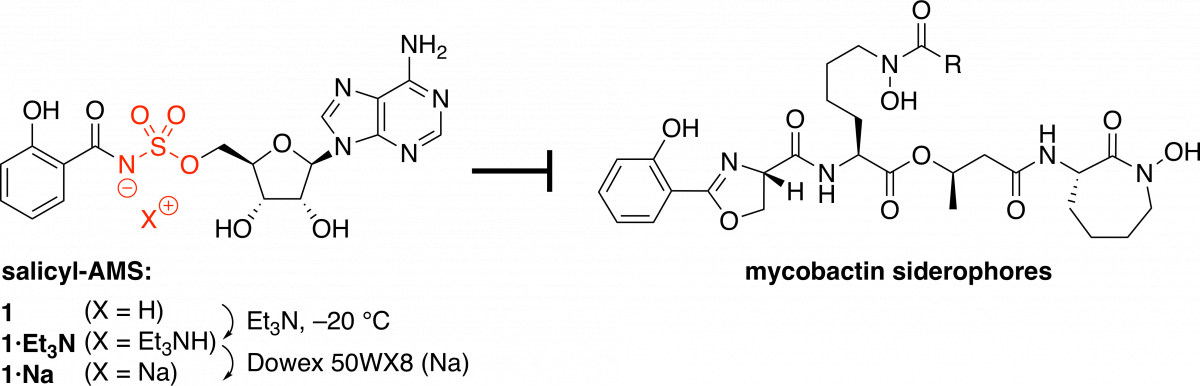
Gram-scale preparation of the antibiotic lead compound salicyl-AMS, a potent inhibitor of bacterial salicylate adenylation enzymes.
Kinarivala, N; Standke, L. C; Guney, T.; Cheng, J.; Naoyoshi, N.; Yasutomi, A.; Tan, D. S.* Method Enzymol. 2020, 638, 69–87.
[ Abstract | PubMed | PMC ]
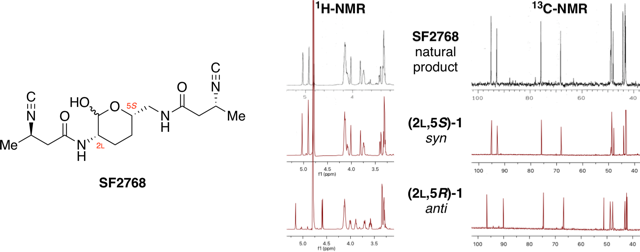
Total synthesis of the bacterial diisonitrile chalkophore SF2768.
Xu, Y.; Tan, D. S.* Org. Lett. 2019, 21, 8731–8735.
[ Abstract | PubMed | PMC ]
Structural basis for adenylation and thioester bond formation in the ubiquitin E1.
Hann, Z. S.; Ji, C.; Olsen, S. K.; Lu, X.; Lux, M. C.; Tan, D. S. *; Lima, C. D.* Proc. Natl. Acad. Sci. U.S.A. 2019, 15475–15484.
[ Abstract | PubMed | PMC ]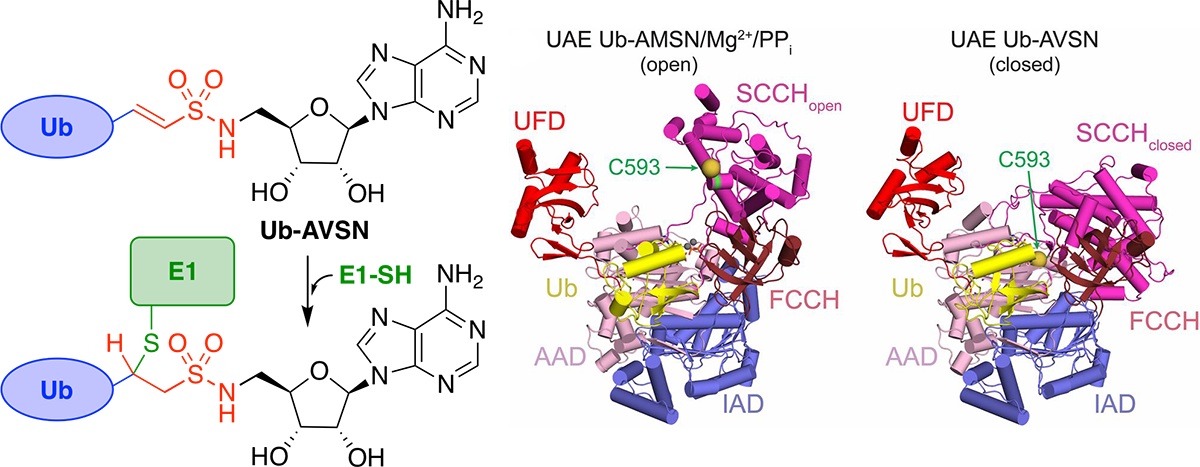
(Highlighted in Proc. Natl. Acad. Sci. USA )
Small-molecule targeting of MUSASHI RNA-binding activity in acute myeloid leukemia.
Minuesa, G.; Albanese, S. K.; Xie, W.; Kazansky, Y.; Worroll, D.; Chow, A.; Schurer, A.; Park, S. M.; Rotsides, C. Z.; Taggart, J.; Rizzi, A.; Naden, L. N.; Chou, T.; Gourkanti, S.; Cappel, D.; Passarelli, M. C.; Fairchild, L.; Adura, C.; Glickman, J. F.; Schulman, J.; Famulare, C.; Patel, M.; Eibl, J. K.; Ross, G. M.; Bhattacharya, S.; Tan, D. S.; Leslie, C. S.; Beuming, T.; Patel, D. J.; Goldgur, Y.; Chodera, J. D.; Kharas, M. G.* Nat Commun 2019, 10, 2691.
[ Abstract | PubMed | PMC ]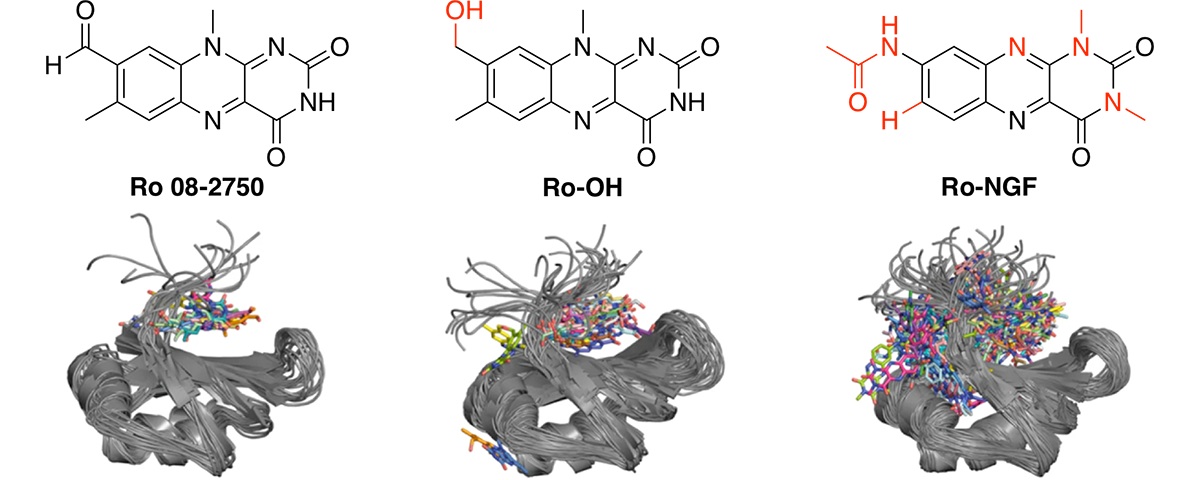
Synthesis of bicyclic ethers by a palladium-catalyzed oxidative cyclization-redox relay-π-allyl-Pd cyclization cascade reaction.
Lux, M. C.; Boby, M. L.; Brooks, J. L.; Tan, D. S.* Chem. Commun. 2019, 55, 7013–7016.
[ Abstract | PubMed | PMC ]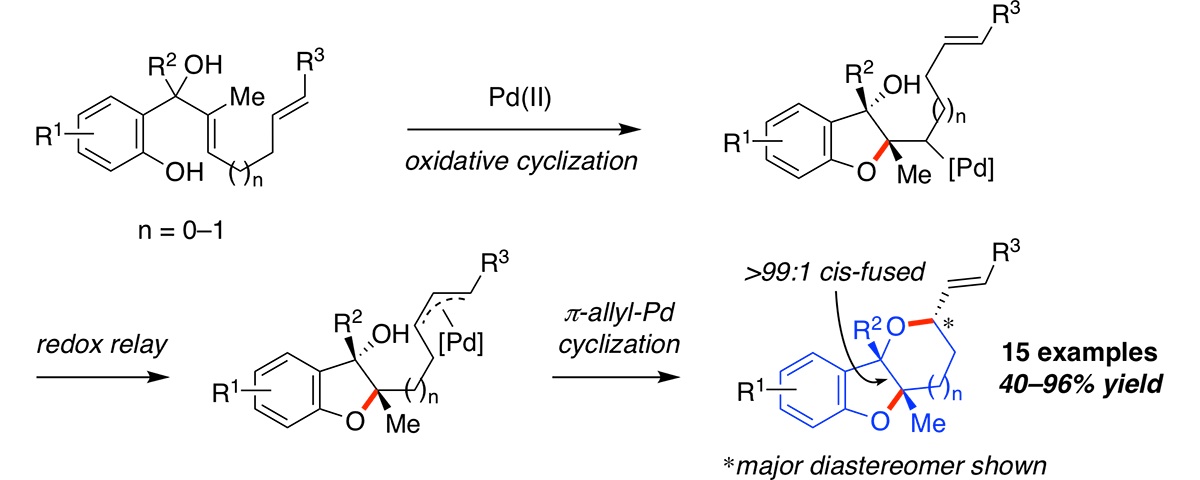
Targeting adenylate-forming enzymes with designed sulfonyladenosine inhibitors.
Lux, M. C.; Standke, L. C.; Tan, D. S.* J. Antibiot. 2019, 72, 325–349.
[ Abstract | PubMed | PMC ]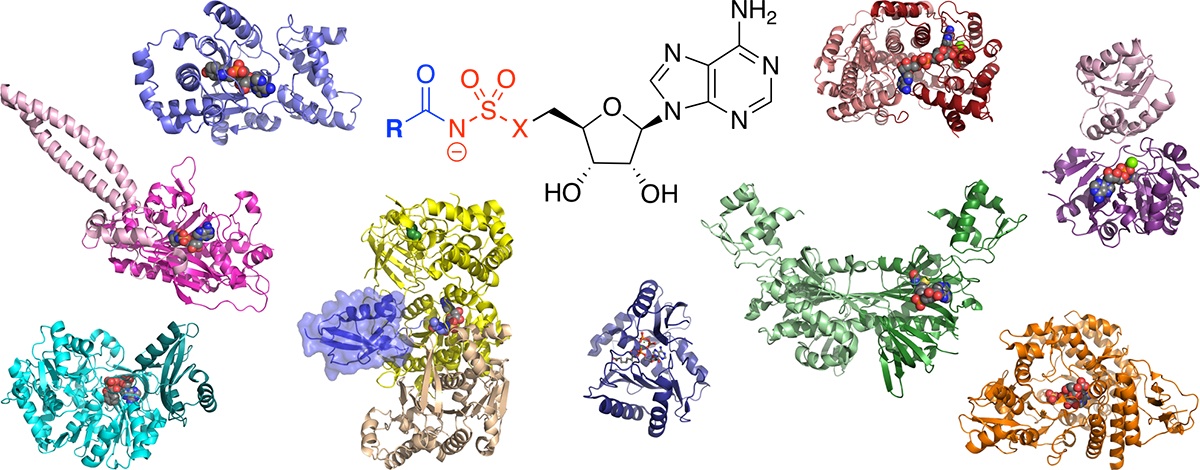
Structure-based design, synthesis, and biological evaluation of non-acyl sulfamate inhibitors of the adenylate-forming enzyme MenE.
Evans, C. E.; Si, Y.; Matarlo, J. S.; Yin, Y.; French, J. B.; Tonge, P. J.*; Tan, D. S.*Biochemistry 2019, 58, 1918–1930.
[ Abstract | PubMed | PMC ]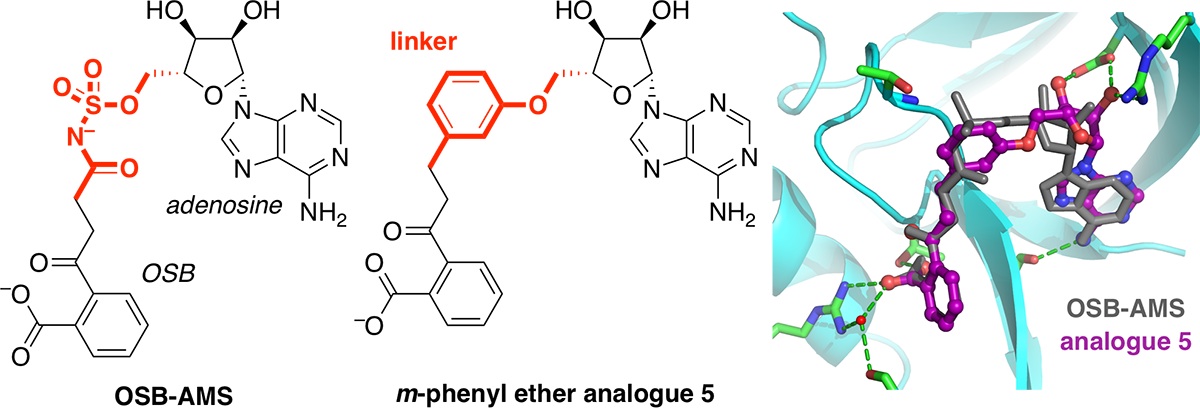
Kinetic analyses of the siderophore biosynthesis inhibitor salicyl-AMS and analogues as MbtA inhibitors and antimycobacterial agents.
Bythrow, G. V.; Mohandas, P.; Guney, T.; Standke, L. C.; Germain, G. A.; Lu, X.; Ji, C.; Levendosky, K.; Chavadi, S. S.; Tan, D. S.*; Quadri, L. E. N.* Biochemistry 2018, 883–847.
[ Abstract | PubMed | PMC ]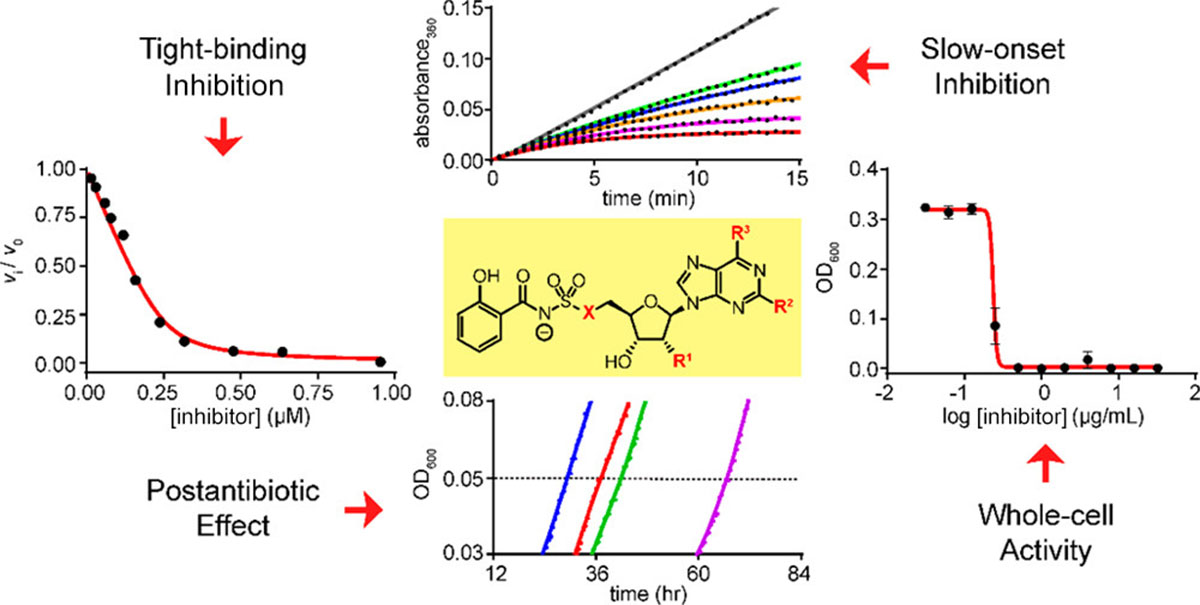
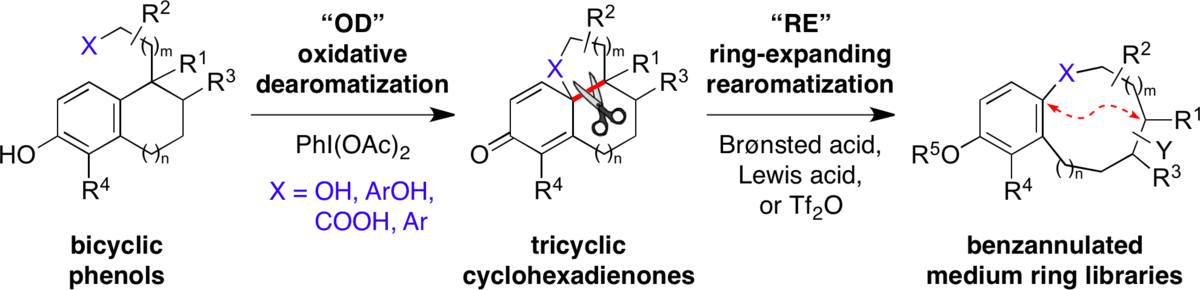
Synthesis of Benzannulated Medium-ring Lactams via a Tandem Oxidative Dearomatization-Ring Expansion Reaction.
Guney, T. †; Wenderski, T. A. †; Boudreau, M. W.; Tan, D. S.* Chem. Eur. J. 2018, in press.
[ Abstract | PubMed |
(Cover article in Chem. Eur. J )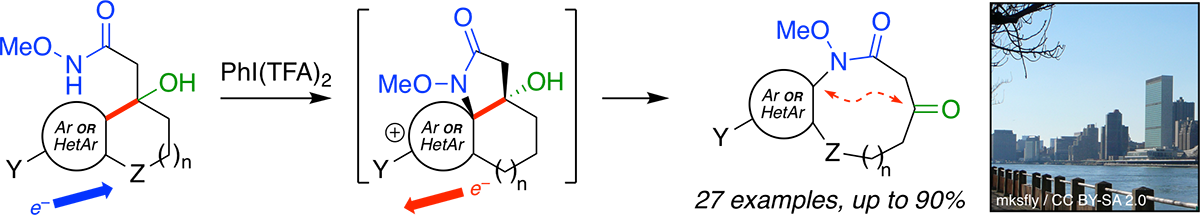
Family-level stereoselective synthesis and biological evaluation of pyrrolomorpholine spiroketal natural product antioxidants
Verano, A. L.; Tan, D. S.* Chem. Sci. 2017, 8, 3687–3693.
[ Abstract | PubMed | PMC ]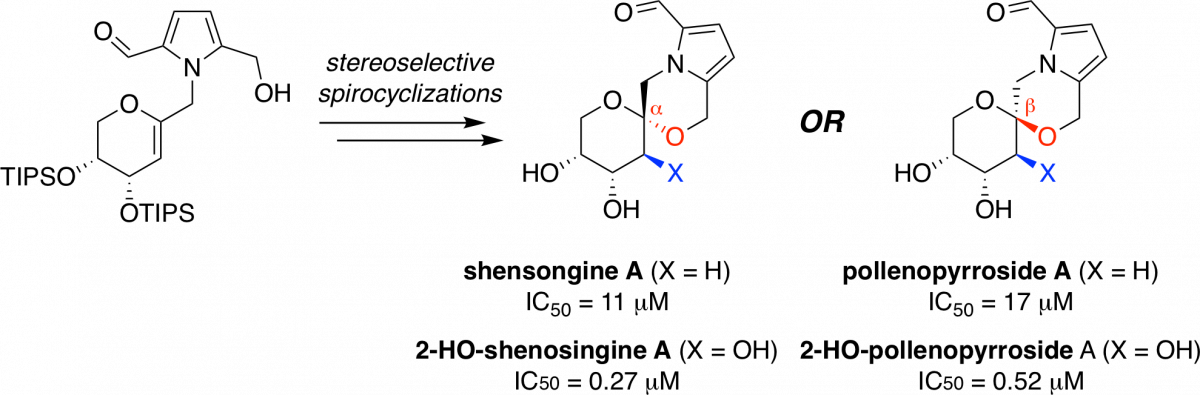
-
Stereocontrolled Synthesis of Spiroketals: An Engine for Chemical and Biological Discovery
Verano, A. L.; Tan, D. S.* Isr. J. Chem. 2017, 57, 279–291.
[ Abstract | PubMed | PMC ]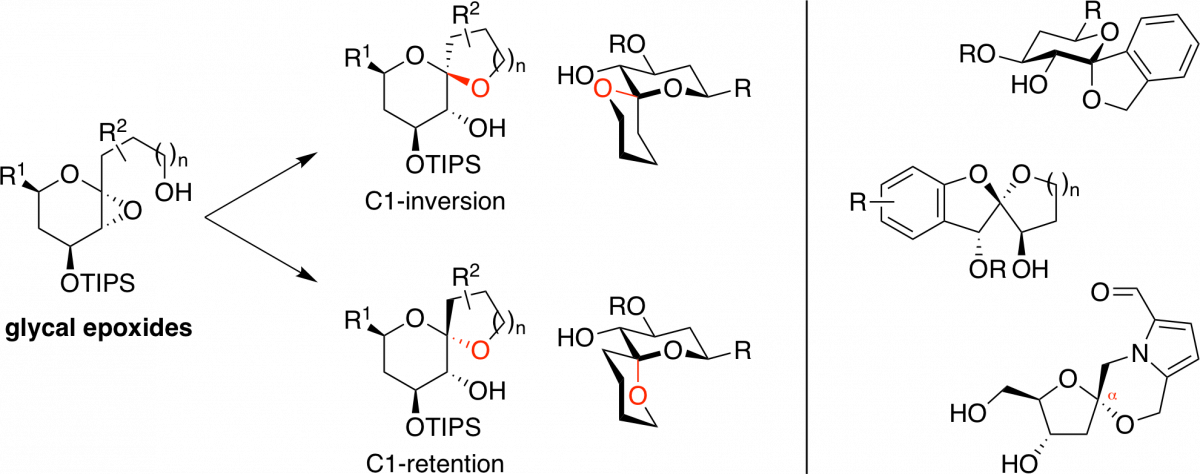
Diastereoselective synthesis of highly substituted tetrahydrofurans by Pd-catalyzed tandem oxidative cyclization-redox relay reactions controlled by intramolecular hydrogen bonding.
Brooks, J. L.; Xu, L.; Wiest, O.; Tan, D. S.* J. Org. Chem. 2017, 82, 57–75.
[ Abstract | PubMed | PMC ]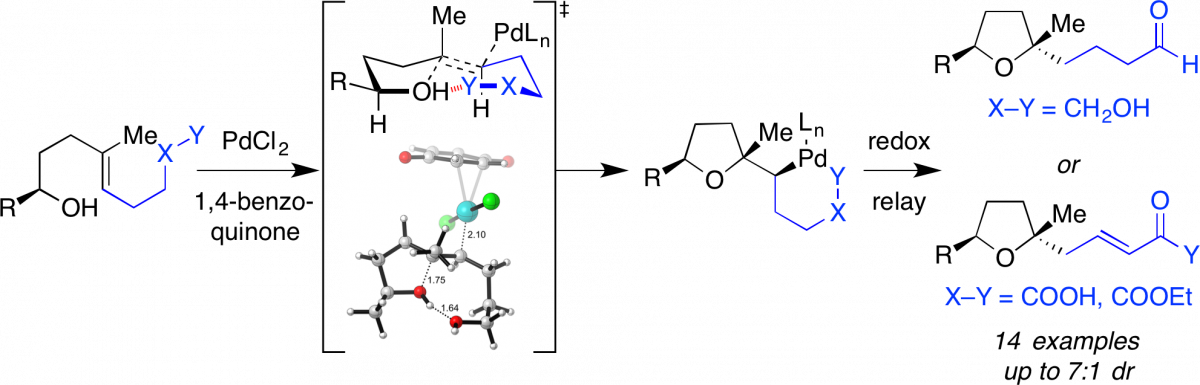
Stereoselective synthesis, docking, and biological evaluation of difluoroindanediol-based MenE inhibitors as antibiotics.
Evans, C. E.; Matarlo, J. S.; Tonge, P. J.*; Tan, D. S.* Org. Lett. 2016, 18, 6384–6387.
[ Abstract | PubMed | PMC ]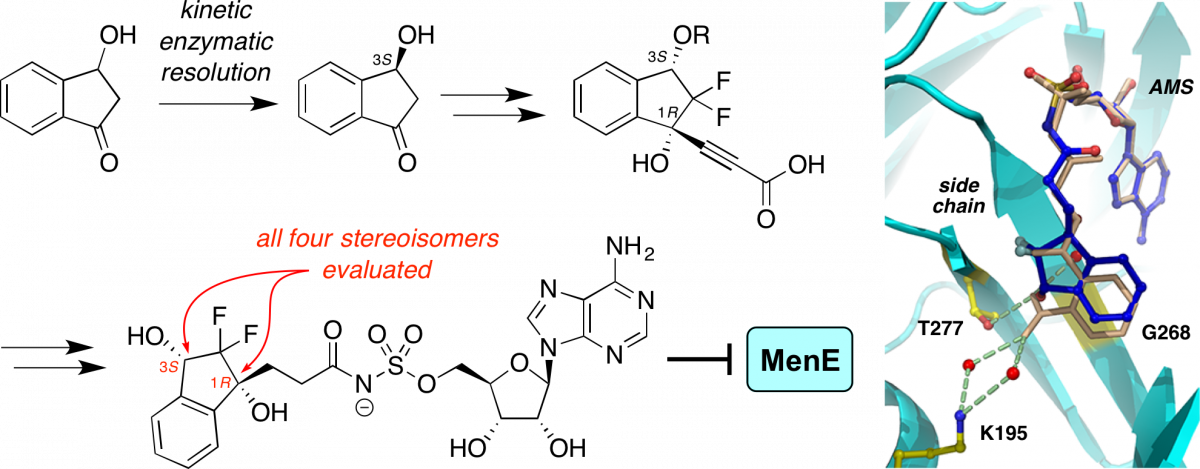
Design, synthesis, and biological evaluation of α-hydroxyacyl-AMS inhibitors of amino acid adenylation enzymes.
Davis, T. D.†; Mohandas, P. †; Chiriac, M. I.; Bythrow, G. V.; Quadri, L. E. N.*; Tan, D. S.* Bioorg. Med. Chem. Lett. 2016, 21, 5340–5345.
[ Abstract | PubMed | PMC ]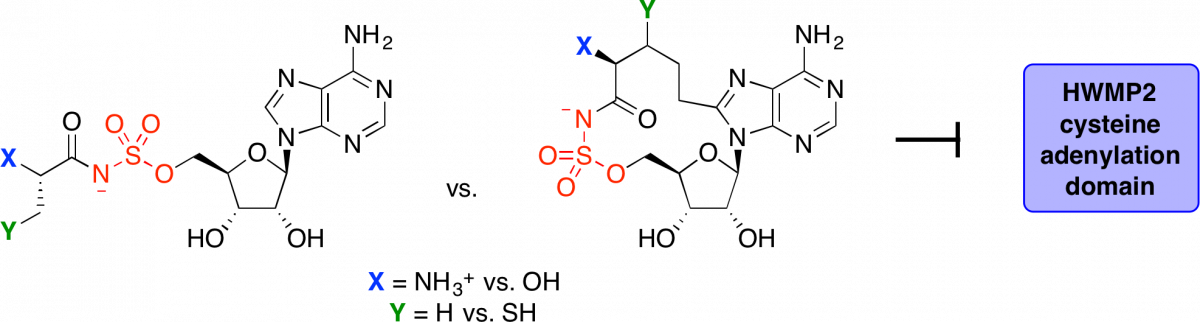
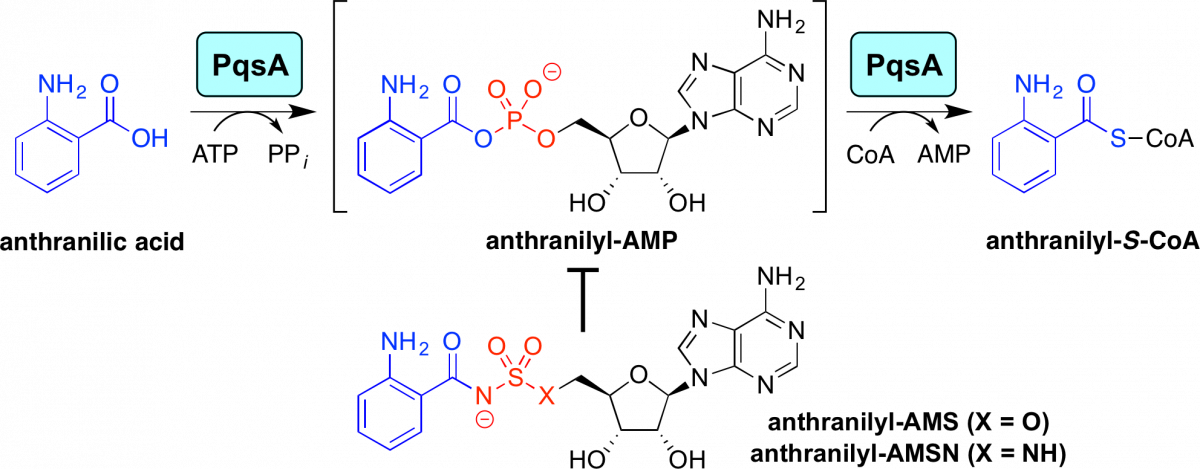
Designed small-molecule inhibitors of the anthranilyl-CoA synthetase PqsA block quinolone biosynthesis in Pseudomonas aeruginosa.
Ji, C.; Sharma, I.; Pratihar, I.; Hudson, L.; Maura, D.; Guney, T.; Rahme, L. G.; Pesci, E. C.; Coleman, J. P.; Tan, D. S.* ACS Chem. Biol. 2016, 11, 3061–3067.
[ Abstract | PubMed | PMC ]
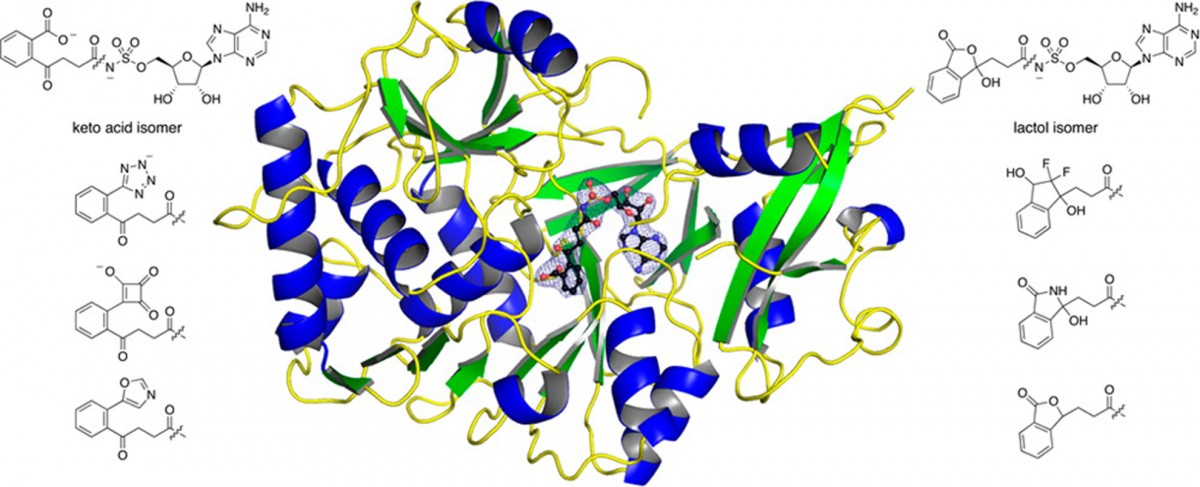
Mechanism of MenE inhibition by acyl-adenylate analogues and discovery of novel antibacterial agents.
Matarlo, J. S.†; Evans, C. E.†; Sharma, I.; Lavaud, L. J.; Ngo, S. C.; Shek, R.; Rajashankar, K. R.; French, J. B.; Tan, D. S.*; Tonge, P. J.* Biochemistry 2015, 54, 6514–6524.
[ Abstract | PubMed | PMC ]
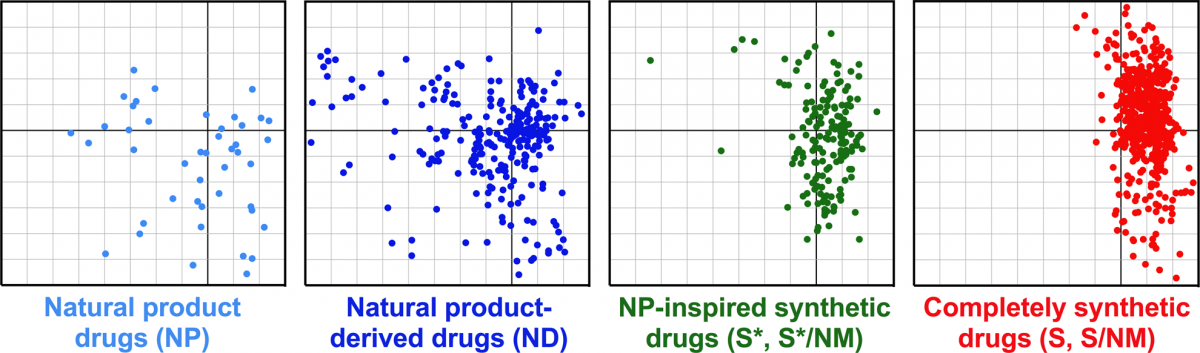
Cheminformatic comparison of approved drugs from natural product versus synthetic origins.
Stratton, C. F.; Newman, D. J.; Tan, D. S.* Bioorg. Med. Chem. Lett. 2015, 25, 4802–4807.
[ Abstract | PubMed | PMC ]
Principal component analysis as a tool for library design: A case study investigating natural products, brand-name drugs, natural product-like libraries, and drug-like libraries.
Wenderski, T. A.; Stratton, C. F.; Bauer, R. A.; Kopp, F.; Tan, D. S.* Methods Mol. Biol. 2015, 1263, 225–242.
[ Abstract | PubMed | PMC ]
General platform for systematic quantitative evaluation of small-molecule permeability in bacteria.
Davis, T. D.; Gerry, C. J.; Tan, D. S.* ACS Chem. Biol. 2014, 9, 2535–2544.
[ Abstract | PubMed | PMC ]
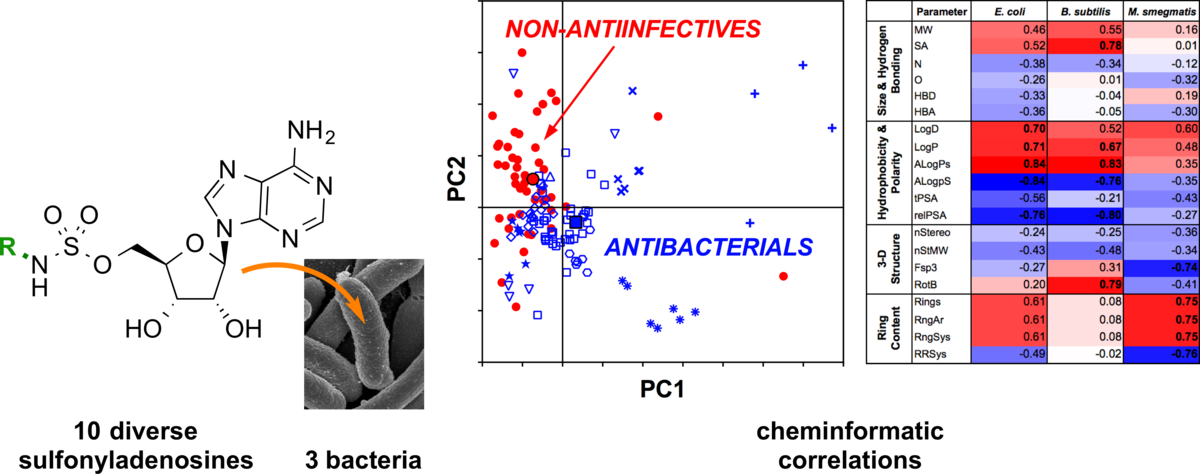
(Highlighted in ACS Chem. Biol.)
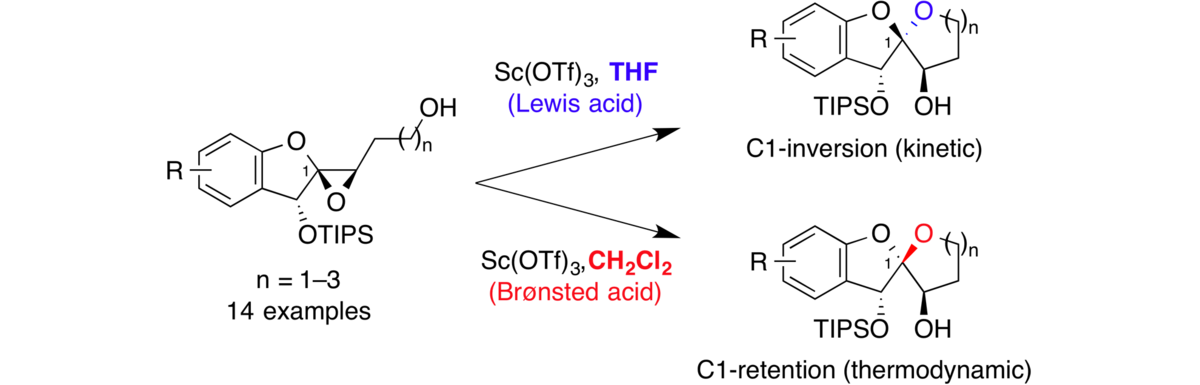
Solvent-dependent divergent functions of Sc(OTf)3 in stereoselective epoxide-opening spiroketalizations.
Sharma, I.; Wurst, J. M.; Tan, D. S.* Org. Lett. 2014, 16, 2474–2477.
[ Abstract | PubMed | PMC ]
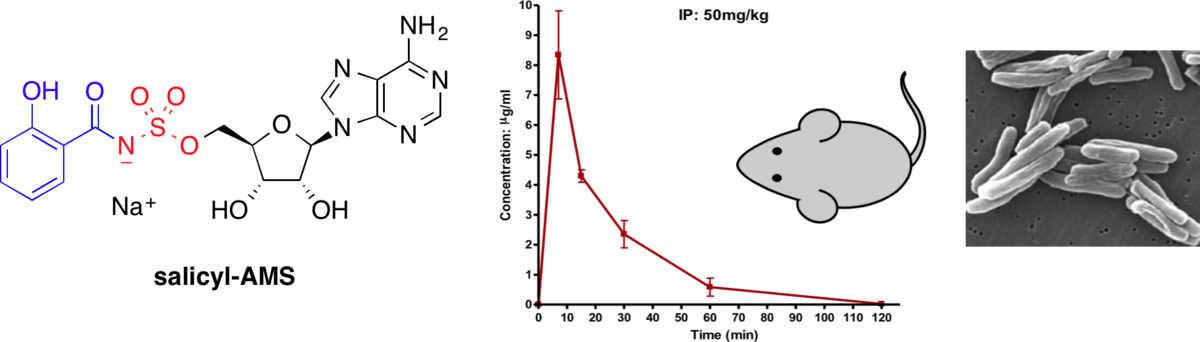
Pharmacokinetic and in vivo efficacy studies of the mycobactin biosynthesis inhibitor salicyl-AMS in mice.
Lun, S.; Guo, H.; Adamson, J.; Cisar, J. S.; Davis, T. D.; Sundaramn Chavadi, S.; Warren, J. D.; Quadri, L. E. N.*; Tan, D. S.*; Bishai, W. R.* Antimicrob. Agents Chemother. 2013, 57, 5138–5140.
[ Abstract | PubMed | PMC ]
Diversifying complexity.
Sharma, I.; Tan, D. S.* Nat. Chem. 2013, 5, 157–158.
[ Abstract | PubMed ]
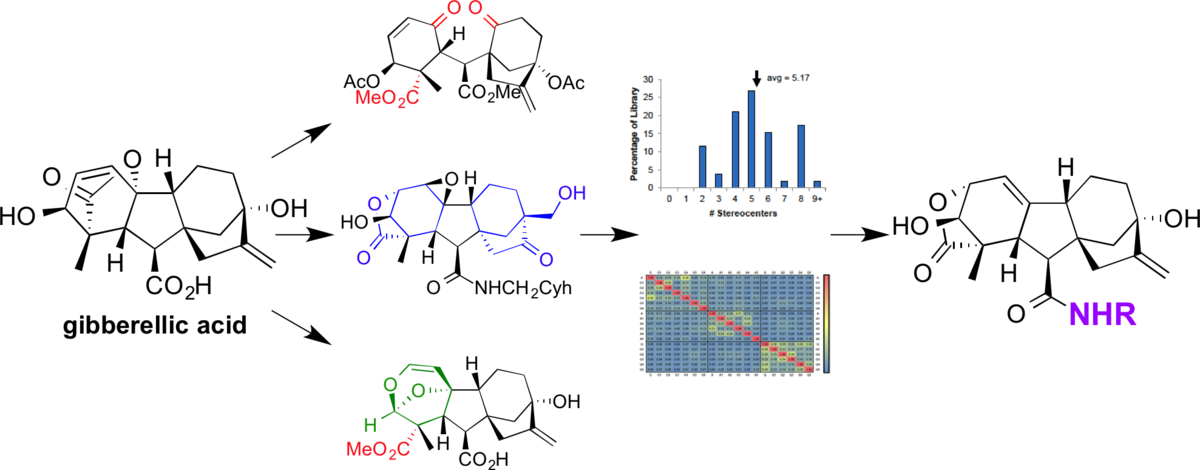
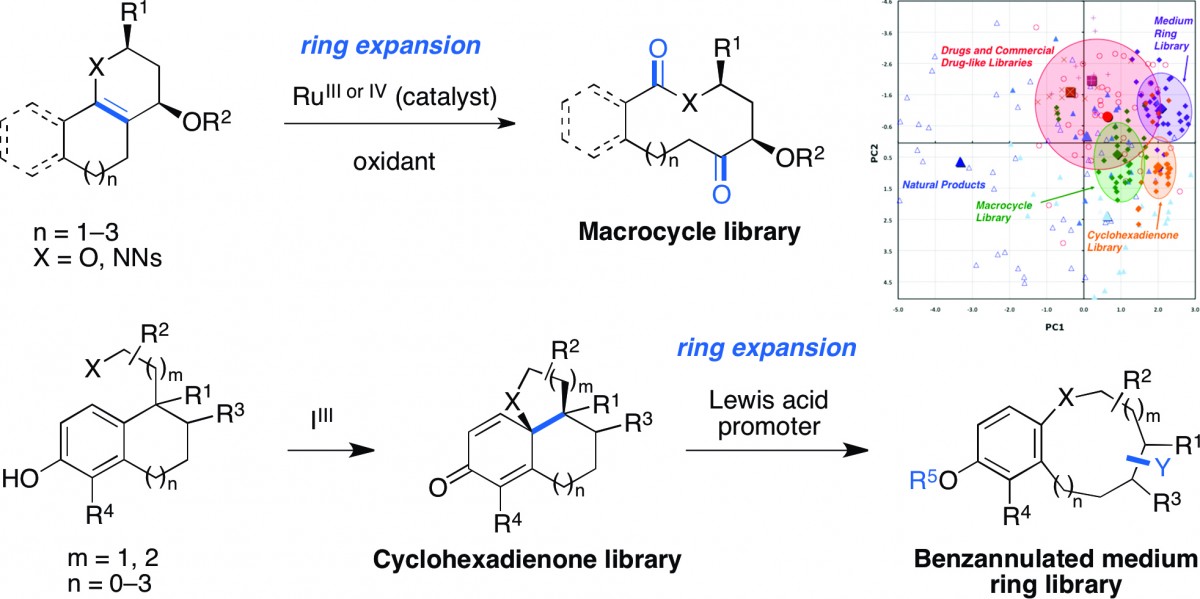
Biomimetic diversity-oriented synthesis of benzannulated medium rings via ring expansion.
Bauer, R. A.; Wenderski, T. A.; Tan, D. S.* Nat. Chem. Biol. 2013, 9, 21–29.
[ Abstract | PubMed | PMC ]
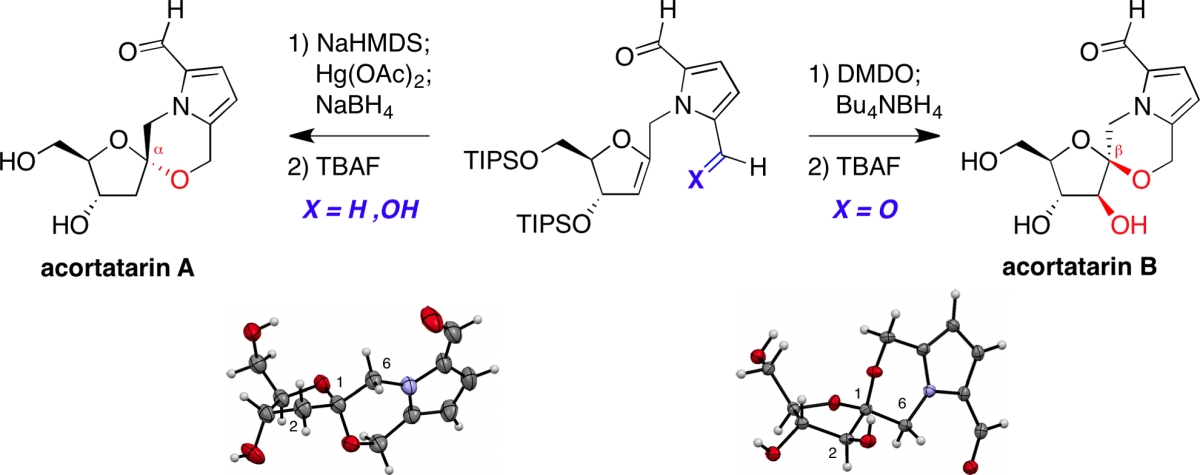
Stereoselective synthesis of acortatarins A and B.
Wurst, J. M.; Verano, A. L.; Tan, D. S.* Org. Lett. 2012, 14, 4442–4445.
[ Abstract | PubMed | PMC ]
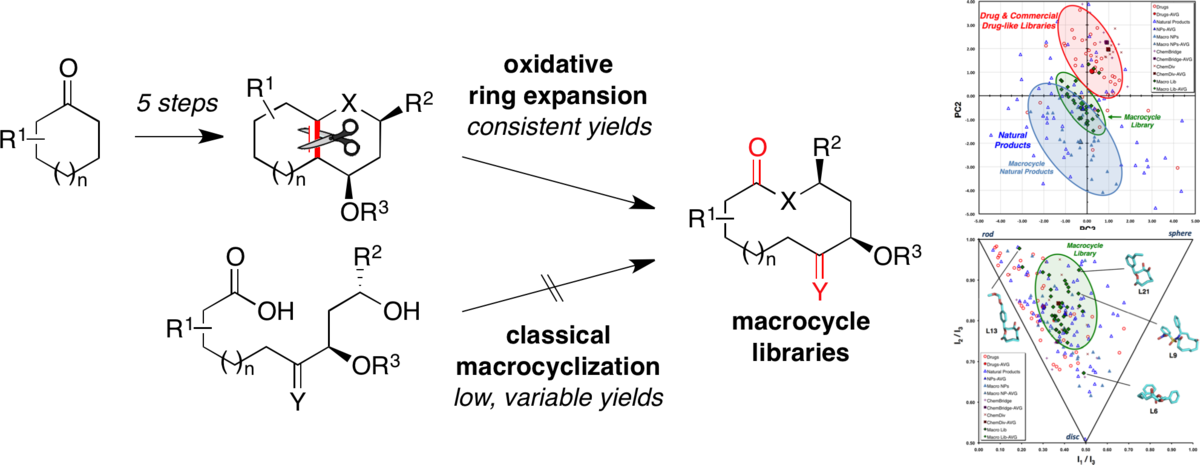
A diversity-oriented synthesis approach to macrocycles via oxidative ring expansion.
Kopp, F.; Stratton, C. F.; Akella, L. B.; Tan, D. S.* Nat. Chem. Biol. 2012, 8, 358–365.
[ Abstract | PubMed | PMC ]
(Highlighted in SciBX )
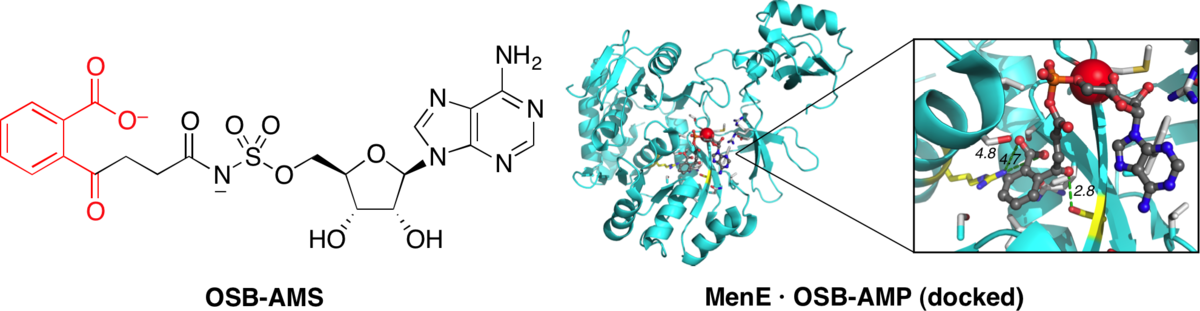
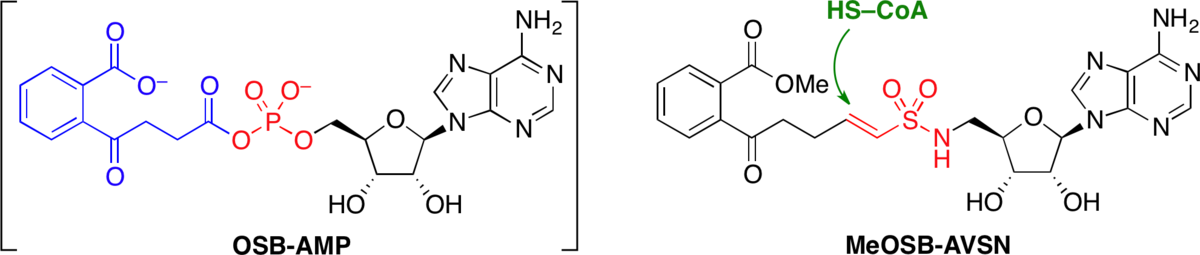
Stable analogues of OSB-AMP: Potent inhibitors of MenE, the o-succinylbenzoate-CoA synthetase from bacterial menaquninone biosynthesis.
Lu, X.; Zhou, R.; Sharma, I.; Li, X.; Kumar, G.; Swaminathan, S.; Tonge, P. J.*; Tan, D. S.* ChemBioChem 2012, 13, 129–136.
[ Abstract | PubMed | PMC ]
Hydrogen-bonding catalysis and inhibition by simple solvents in the stereoselective kinetic epoxide-opening spirocyclization of glycal epoxides to form spiroketals.
Wurst, J. M.; Liu, G.; Tan, D. S.* J. Am. Chem. Soc. 2011, 133, 7916–7925.
[ Abstract | PubMed | PMC ]
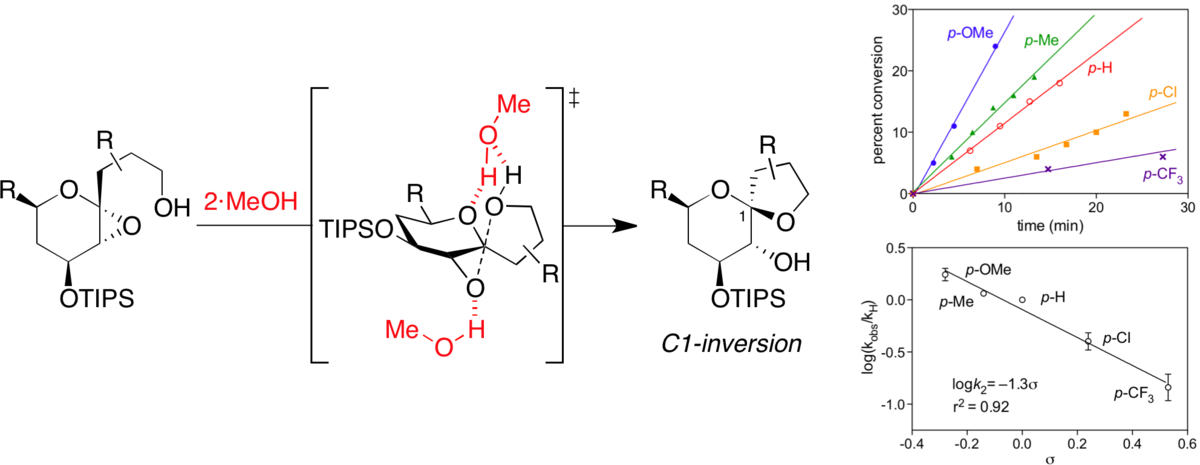
Solid-phase synthesis and chemical space analysis of a 190-membered alkaloid/terpenoid-like library.
Moura-Letts, G.; DiBlasi, C. M.; Bauer, R. A.; Tan, D. S.* Proc. Natl. Acad. Sci. USA 2011, 108, 6745–6750.
[ Abstract | PubMed | PMC ]
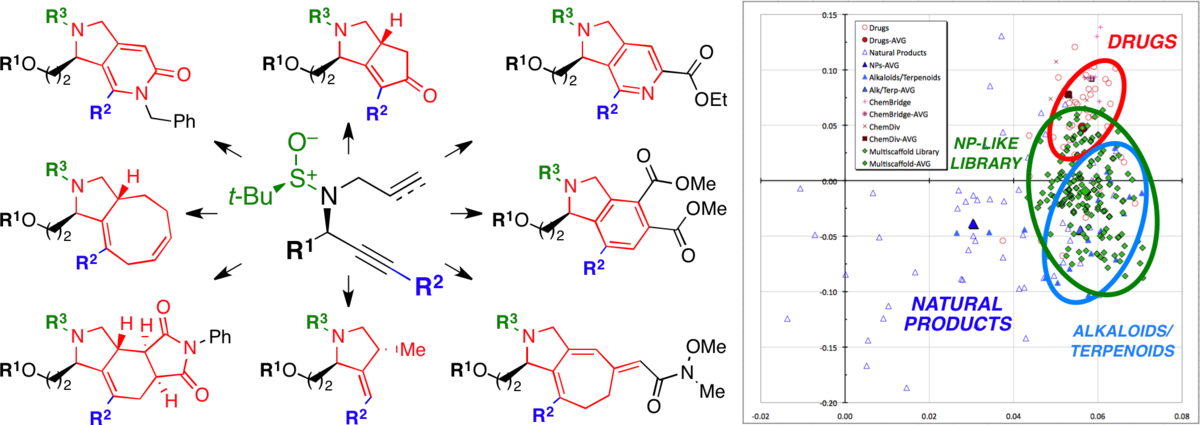
The tert-butylsulfinamide lynchpin in transition-metal-mediated multiscaffold library synthesis.
Bauer, R. A.; DiBlasi, C. M.; Tan, D. S.* Org. Lett. 2010, 12, 2084–2087.
[ Abstract | PubMed | PMC ]
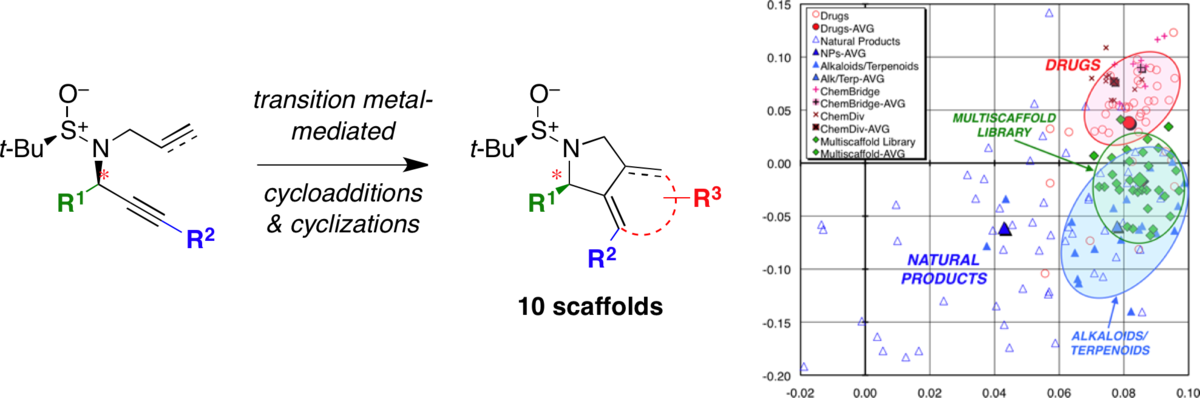
Expanding the range of ’druggable’ targets with natural product-based libraries: An academic perspective.
Bauer, R. A.; Wurst, J. M.; Tan, D. S.* Curr. Opin. Chem. Biol. 2010, 14, 308–314.
[ Abstract | PubMed | PMC ]
Active site remodelling accompanies thioester bond formation in the SUMO E1.
Olsen, S. K.; Capili. A. D.; Lu, X.; Tan, D. S.*; Lima, C. D.* Nature 2010, 463, 906–912.
[ Abstract | PubMed | PMC ]
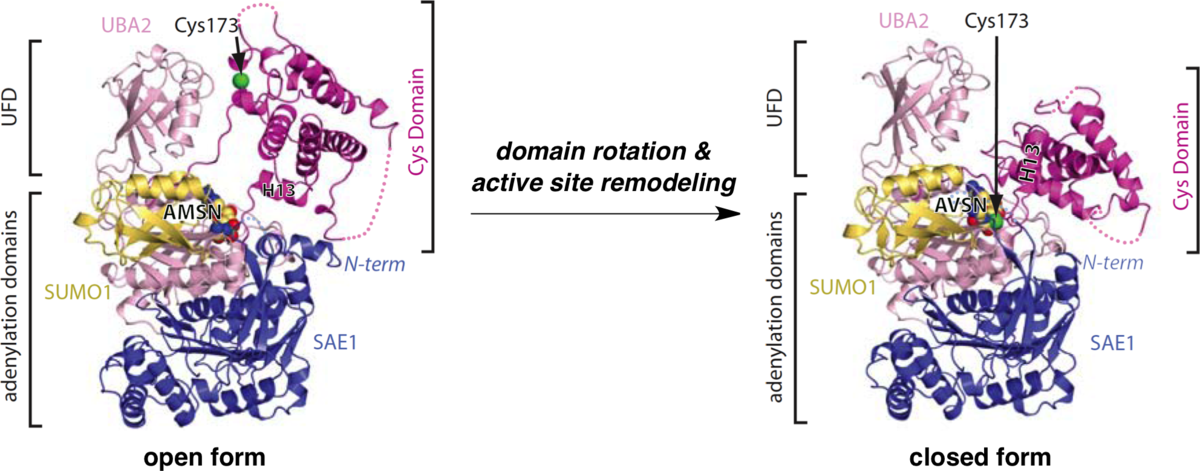
(Highlighted in Nature, Chem. Eng. News, Nat. Rev. Mol. Cell Biol., Nat. Chem. Biol., Structure, ACS Chem. Biol., and Faculty of 1000 Biology )
Designed semisynthetic protein inhibitors of Ub/Ubl E1 activating enzymes.
Lu, X.; Olsen, S. K.; Capili, A. D.; Cisar, J. S.; Lima, C. D.*; Tan, D. S.* J. Am. Chem. Soc. 2010, 132, 1748–1749.
[ Abstract | PubMed | PMC ]
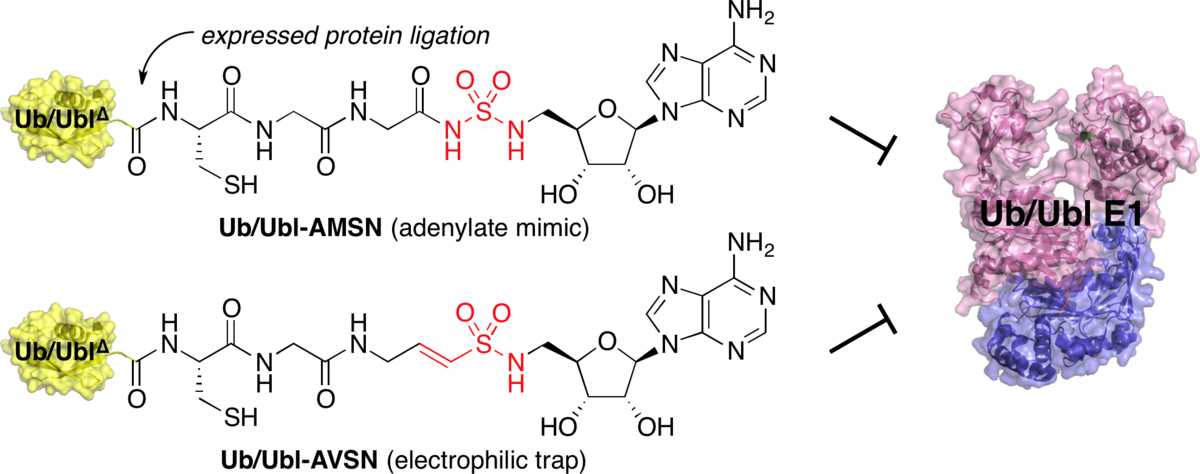
(Highlighted in Chem. Eng. News, Nat. Rev. Mol. Cell Biol., ACS Chem. Biol., and Faculty of 1000 Biology )
Stereoselective synthesis of benzannulated spiroketals: Influence of the aromatic ring on reactivity and conformation.
Liu, G.; Wurst, J. M.; Tan, D. S.* Org. Lett. 2009, 11, 3670–3673.
[ Abstract | PubMed | PMC ]
Mechanism-based inhibitors of MenE, an acyl-CoA synthetase involved in bacterial menaquinone biosynthesis.
Lu, X.; Zhang, H.; Tonge, P. J.*; Tan, D. S.* Bioorg. Med. Chem. Lett. 2008, 18, 5963–5966.
[ Abstract | PubMed | PMC ]
Small molecule inhibition of microbial natural product biosynthesis – An emerging antibiotic strategy.
Cisar, J. S.; Tan, D. S.* Chem. Soc. Rev. 2008, 37, 1320–1329.
[ Abstract | PubMed | PMC ]
Mycobacterial phenolic glycolipid virulence factor biosynthesis: Mechanism and small-molecule inhibition of polyketide chain initiation.
Ferreras, J. A.; Stirrett, K. L.; Lu, X.; Ryu, J.-S.; Soll, C. E.; Tan, D. S.; Quadri, L. E. N.* Chem. Biol. 2008, 15, 51–61.
[ Abstract | PubMed | PMC ]
(Highlighted in Chem. Biol. )
Exploiting ligand conformation in selective inhibition of non-ribosomal peptide synthetase amino acid adenylation with designed macrocyclic small molecules.
Cisar, J. S.; Ferreras, J. A.; Soni, R. K.; Quadri, L. E. N.*; Tan, D. S.* J. Am. Chem. Soc. 2007, 129, 7752–7753.
[ Abstract | PubMed | PMC ]
(Highlighted in Faculty of 1000 Biology )
A unified synthetic approach to polyketides having both skeletal and stereochemical diversity.
Shang, S.; Iwadare, H.; Macks, D. E.; Ambrosini, L. M.; Tan, D. S.* Org. Lett. 2007, 9, 1895–1898.
[ Abstract | PubMed | PMC ]
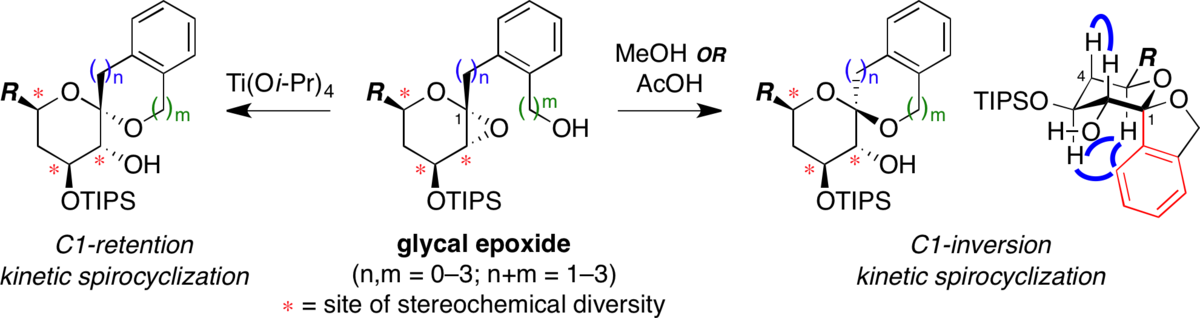
Stereocontrolled synthesis of spiroketals via Ti(Oi-Pr)4-mediated kinetic spirocyclization of glycal epoxides with retention of configuration.
Moilanen, S. B.; Potuzak, J. S.; Tan, D. S.* J. Am. Chem. Soc. 2006, 128, 1792–1793.
[ Abstract | PubMed | PMC ]
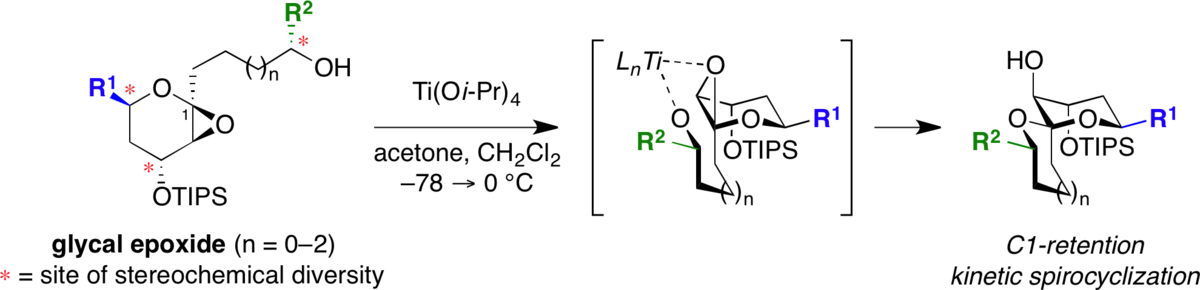
(Highlighted in Nature )
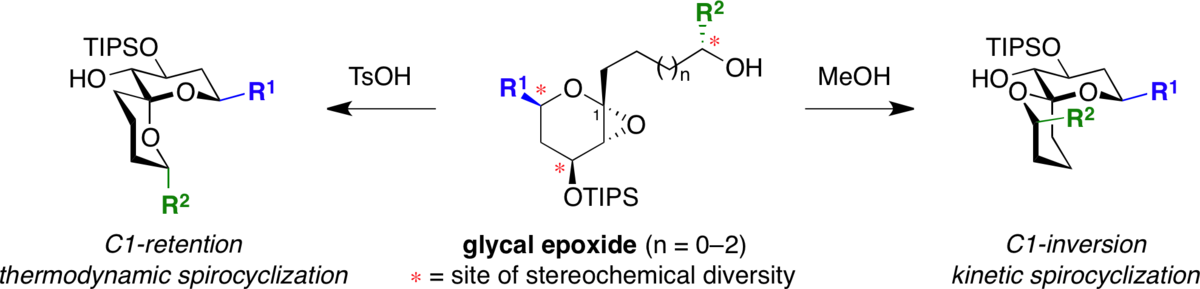
Stereocontrolled synthesis of spiroketals via a remarkable methanol-induced kinetic spirocyclization reaction.
Potuzak, J. S.; Moilanen, S. B.; Tan, D. S.* J. Am. Chem. Soc. 2005, 127, 13796–13797.
[ Abstract | PubMed ]
Diversity-oriented synthesis: Exploring the intersections between chemistry and biology.
Tan, D. S.* Nat. Chem. Biol. 2005, 1, 74–84.
[ Abstract | PubMed ]
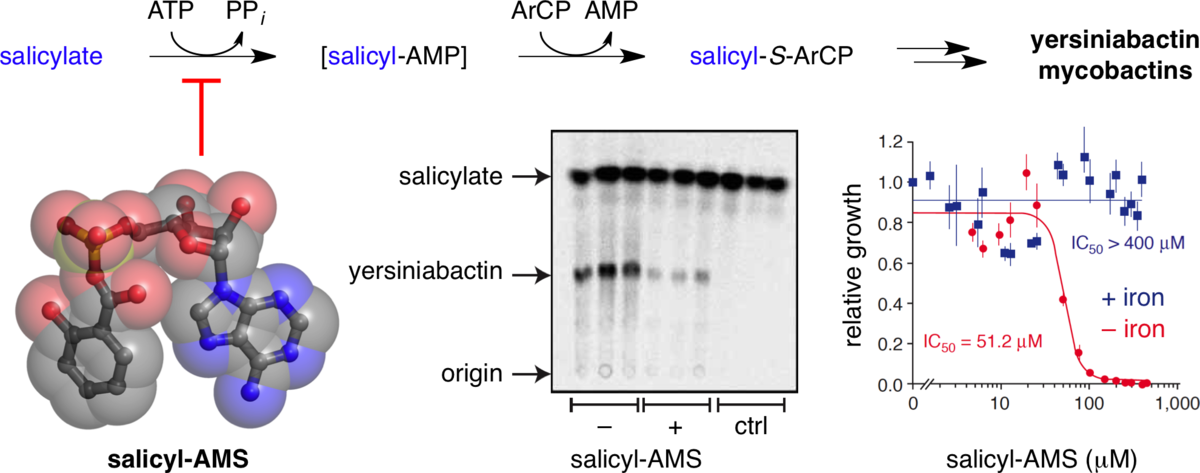
Small-molecule inhibition of siderophore biosynthesis in Mycobacterium tuberculosis and Yersinia pestis.
Ferreras, J. A.; Ryu, J.-S.; Di Lello, F.; Tan, D. S.*, Quadri, L. E. N.* Nat. Chem. Biol. 2005, 1, 29–32.
[ Abstract | PubMed ]
(Highlighted in Nature, Nat. Chem. Biol., Chem. Eng. News., and Mercosur Económico )
Advancing chemistry and biology through diversity-oriented synthesis of natural product-like libraries.
Shang, S.; Tan, D. S.* Curr. Opin. Chem. Biol. 2005, 9, 248–258.
[ Abstract | PubMed ]
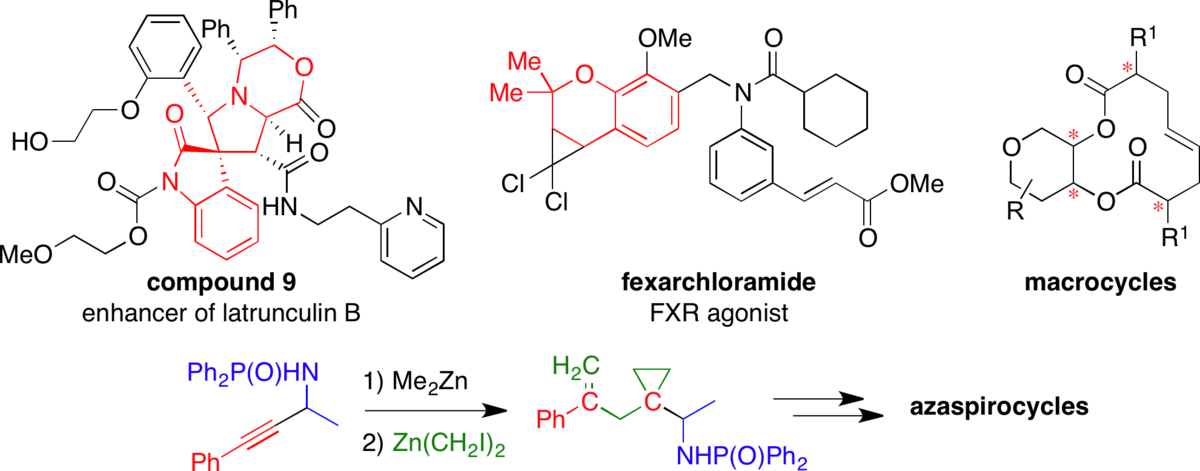
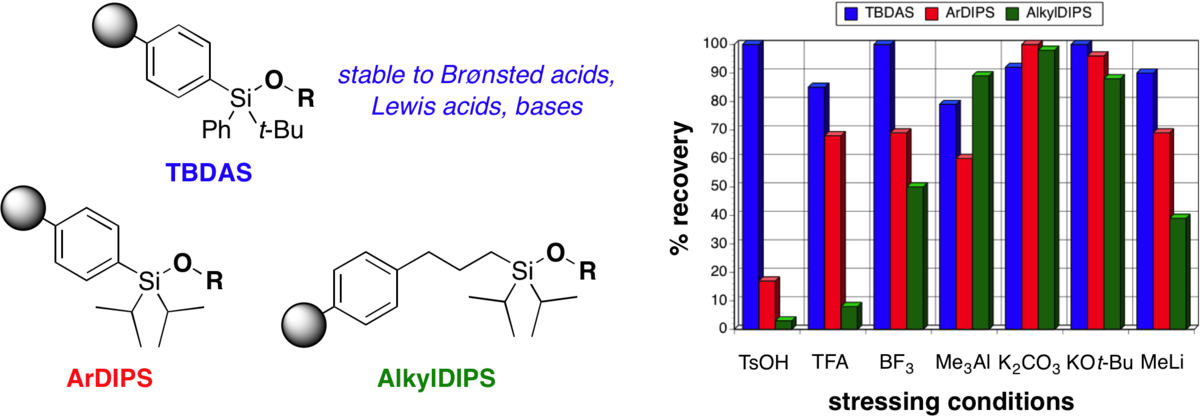
An acid-stable tert-butyldiarylsilyl (TBDAS) linker for solid-phase organic synthesis.
DiBlasi, C. M.; Macks, D. E.; Tan, D. S.* Org. Lett. 2005, 7, 1777–1780.
[ Abstract | PubMed ]
(Highlighted in Lett. Org. Chem. [PDF] )
Enantioselective synthesis of erythro-4-deoxyglycals as scaffolds for target- and diversity-oriented synthesis: New insights into glycal reactivity.
Moilanen, S. B.; Tan, D. S.* Org. Biomol. Chem. 2005, 3, 798–803.
[ Abstract | PubMed ]

Current progress in natural product-like libraries for discovery screening.
Tan, D. S.* Comb. Chem. High-Throughput Screen. 2004, 7, 631–643.
[ Abstract | PubMed ]
Synthesis of C1-alkyl and C1-acylglycals from glycals using a B-alkyl Suzuki–Miyaura cross coupling approach.
Potuzak, J. S.; Tan, D. S.* Tetrahedron Lett. 2004, 45, 1797–1801.
[ Abstract ]
Discovery and applications of small molecule probes for studying biological processes.
Potuzak, J. S.; Moilanen, S. B.; Tan, D. S.* Biotechnol. Genet. Eng. Rev. 2004, 21, 11–78.
[PDF | PubMed ]
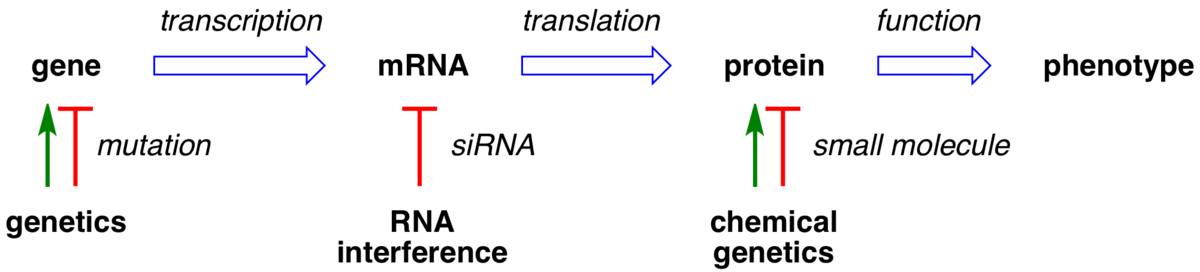
Sweet surrender to chemical genetics.
Tan, D. S.* Nat. Biotechnol. 2002, 20, 561–563.
[ Abstract | PubMed ]
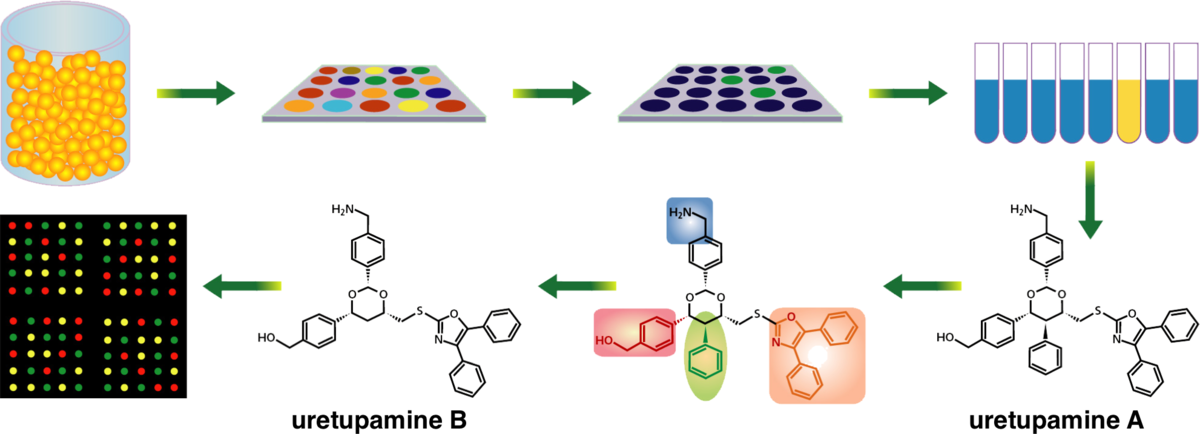
Canvass: A crowd-sourced, natural-product screening library for exploring biological space.
Kearney, S. E. et al. Verano, A. L.; Tan, D. S.; Rohde, J. M.* ACS. Cent. Sci. 2018, 4, 1727–1741.
[ Abstract |
PubMed |
PMC ]
Postdoctoral Publications
Total synthesis of guanacastepene A: A route to enantiomeric control.
Mandal, M.; Yun, H.; Dudley, G. B.; Lin, S.; Tan, D. S.; Danishefsky, S. J.* J. Org. Chem. 2005, 70, 10619–10637.
[ Abstract | PubMed ]
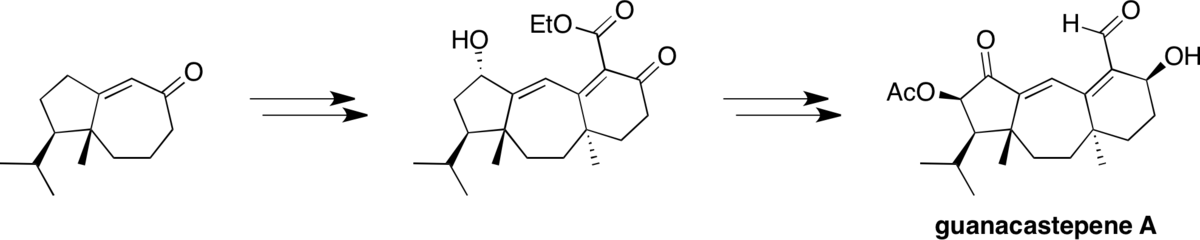
(Featured on the Cover)
Synthesis of the functionalized tricyclic skeleton of guanacastepene A: A tandem epoxide-opening β-elimination/Knoevenagel cyclization.
Tan, D. S.; Dudley, G. B; Danishefsky, S. J.* Angew. Chem., Int. Ed. 2002, 41, 2185–2188.
[ Abstract | PubMed ]
A stereoselective route to guanacastepene A through a surprising epoxidation.
Lin, S.; Dudley, G. B.; Tan, D. S.; Danishefsky, S. J.* Angew. Chem., Int. Ed. 2002, 41, 2188–2191.
[ Abstract | PubMed ]

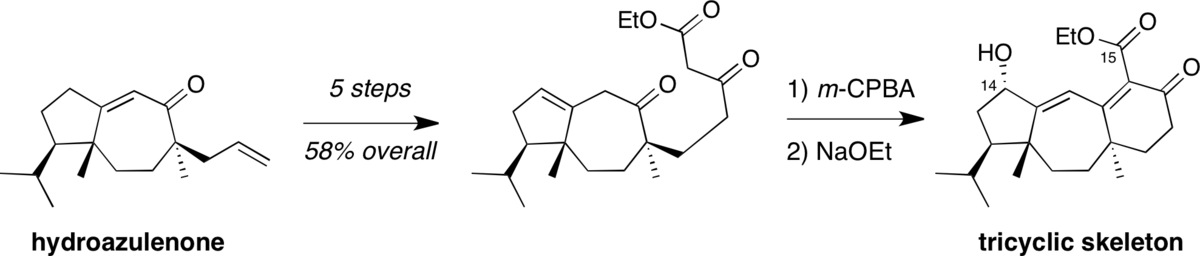
Remarkable stereoselectivity in the alkylation of a hydroazulenone: Progress toward the total synthesis of guanacastepene.
Dudley, G. B.; Tan, D. S.; Kim, G.; Tanski, J. M.; Danishefsky, S. J.* Tetrahedron Lett. 2001, 42, 6789–6791.
[ Abstract ]
Graduate Publications
A mercury-catalyzed transetherification cyclization leading to fused cyclic polyethers.
Tan, D. S.; Schreiber, S. L.* Tetrahedron Lett. 2000, 41, 9509–9513.
[ Abstract ]
Ligand discovery using encoded combinatorial libraries.
Tan, D. S.*; Burbaum, J. J.* Curr. Opin. Drug Discovery Dev. 2000, 3, 439–453.
[Abstract]
Synthesis and preliminary evaluation of a library of polycyclic small molecules for use in chemical genetic assays.
Tan, D. S.; Foley, M. A.; Stockwell, B. R.; Shair, M. D.; Schreiber, S. L.* J. Am. Chem. Soc. 1999, 121, 9073–9087.
[ Abstract ]
Stereoselective synthesis of over two million compounds having structural features both reminiscent of natural products and compatible with miniaturized cell-based assays.
Tan, D. S.; Foley, M. A.; Shair, M. D.; Schreiber, S. L.* J. Am. Chem. Soc. 1998, 120, 8565–8566.
[ Abstract ]

(Highlighted in Science, Chem. Eng. News. )
Undergraduate Publication
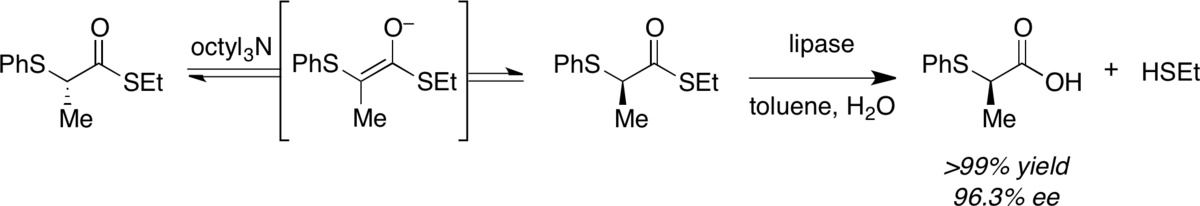
Enzymatic resolution coupled with substrate racemization using a thioester substrate.
Tan, D. S.; Günter, M. M.; Drueckhammer, D. G.* J. Am. Chem. Soc. 1995, 117, 9093–9094.
[ Abstract ]