My research is currently focused in understanding the biology underlying the decline and recovery of the thymus and bone marrow after immune insults, and use this knowledge to individuate therapeutic targets for the regeneration of the hematopoietic system in immunocompromised patients.
Optimal recovery of hematopoietic function is a critical process in several clinical situations such as after cytoreductive chemo- or radiation therapy, infection, shock, as well as after radiation injury. Prolonged immune suppression has consequences at multiple levels including increased opportunistic infections, malignant relapse, morbidity and mortality. Therefore, development of strategies to improve the regeneration of hematopoietic system and reconstitution of the peripheral T cell pool represents a significant clinical challenge with the potential to improve overall outcome in immunocompromised recipients.
Regeneration of Thymic Functionality

The thymus, which is primarily responsible for generating T cells with a diverse T cell receptor (TCR) repertoire, undergoes a severe involution with age.This decline in thymic function has been in part attributed to the increase in sex steroids after puberty, and their removal promotes thymic regeneration. We have recently show that one of the mechanisms by which sex steroids regulate thymic function is through direct modulation of the Notch ligand Delta-like 4 (Dll4), a critical gene involved in T cell commitment and differentiation. We found that administration of testosterone decreased the expression of Dll4 specifically in cortical thymic epithelial cells (cTECs).Using a computational approach, and subsequently validated by ChIP studies, we found that the androgen receptor (AR) could directly bind and negatively regulate the promoter of Dll4. Consistent with this, sex steroid ablation (SSA), using a novel class of luteinizing hormone-releasing hormone receptor antagonists (LHRH-Ant), promoted upregulation of Dll4 in cTECs and its downstream target genes Hes1, Ptcra and CD25 in double-negative 3 thymocytes. Importantly, we also found that LHRH-Ant treatment bypassed the surge in sex steroids caused by LHRH-R agonists - the gold standard for clinical SSA – thereby facilitating more rapid promotion of thymopoiesis (Velardi et al. Journal of Experimental Medicine 2014 Nov 17;211(12):2341-9).
Recovery of thymic function is a critical process that allows for the renewal of immune competence following hematopoietic insults such as infection, stress and cytotoxic interventions commonly used in the treatment of cancer patients. Using clinically relevant mouse models of immune injury, we found that the LHRH-Ant promoted faster thymic regeneration after total body irradiation resulting in faster peripheral T cell reconstitution and enhanced viral clearance (Velardi et al. Journal of Experimental Medicine 2014 Nov 17;211(12):2341-9).
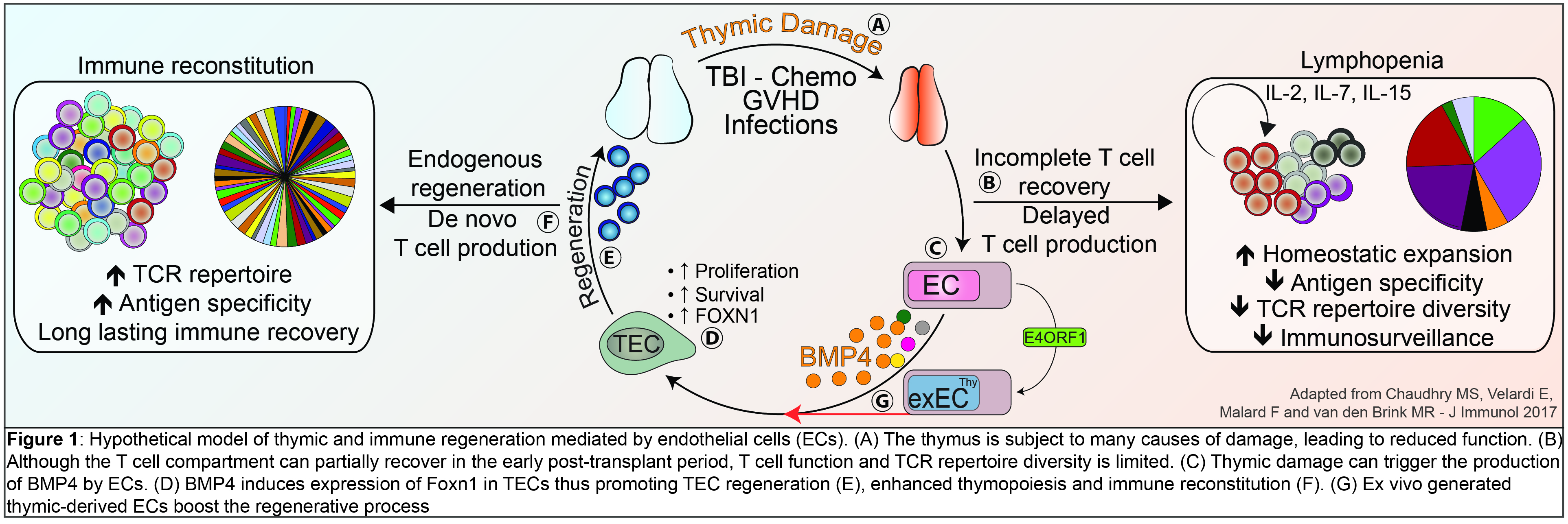
More recently, we have uncovered a previously unrecognized pathway of thymic regeneration. We can demonstrate that thymic endothelial cells (ECs) are actively involved in thymic reconstitution after insults through their production of Bone morphogenetic protein 4 (BMP4). We found that ECs represent a damage-resistant niche within the thymus and, following thymic insults, ECs increase the production of BMP4, which acts on thymic epithelial cells (TECs) to increase the expression of Foxn1 - a key transcription factor involved in TEC proliferation, regeneration and thymus regrowth. These effects promote thymic reconstitution after injury. These studies lay the foundation for the development of novel strategies to enhance thymic function in patients whose immune system has been decimated due to aging, infection, or cytotoxic cancer treatments. (Wertheimer*, Velardi*, Tsai* et al Science Immunology. 2018 Jan 12;3(19)) .
Boosting hematopoietic stem cell recovery after injuries
Nature Medicine - February 2018 Volume 24 No 2
In a study, recently published in Nature Medicine, we identified a novel strategy to protect hematopoietic stem cells (HSCs) from exhaustion and promote hematopoietic regeneration after myeloablative treatments. HSCs are responsible for maintaining continuous blood production throughout life and to initiate hematopoietic recovery after periods of stress or injury. HSC pool size is tightly regulated by extrinsic and intrinsic signaling pathways. Here we demonstrate that the most primitive HSCs express high levels of the LH receptor and that LH stimulation promotes HSC expansion. Conversely, ablation of LH mediates HSC quiescence and survival after hematopoietic insults. To elucidate the molecular changes induced by LH inhibition, we assessed the expression of genes associated with quiescence, proliferation, DNA damage and apoptosis in HSC after SL-TBI. We found that LH suppression promoted higher levels of Hes1, Cdkn1a (p21), Bcl2 and Mcl1, genes previously identified to enforce HSC quiescence and survival. It has been previously showing that promoting quiescence early after hematopoietic insults can mitigate HSC exhaustion and, ultimately, promote hematopoietic recovery and mouse survival. Mice receiving LHRH-Ant 24h after lethal TBI (840cGy) exposure showed a significant increase in survival compared to control animals. In fact, LH suppression spared LT-HSCs from radiation toxicity thus promoting hematopoietic recovery. Our study reveals an unexpected role of LH in regulating HSC homeostasis and also demonstrates that the pharmacological inhibition of LH represents a potent medical countermeasure against radiation exposure. (Velardi et al, Nature Medicine. 2018 Jan 8. doi: 10.1038/nm.4470).
Awards
- Amy Strelzer Manasevit Research Program Award (2018)
- ASH Abstract Achievement Award (58th ASH Annual Meeting in San Diego, CA) (2016)
- Immunology and Microbial Pathogenesis (IMP) Scientific Retreat Publication Award (2015)
- AAI Trainee Abstract Award (The American Association of Immunologists Annual Meeting – New Orleans, LA) (2015)
- ASH Abstract Achievement Award (55th ASH Annual Meeting), New Orleans, LA (2014)
- American Society for Blood and Marrow Transplantation New Investigator Award (2013)
- Italian Foundation for Cancer Research Award for Abroad Research (2011)
- Italian Society of Pharmacology Award for Abroad Research (2011)