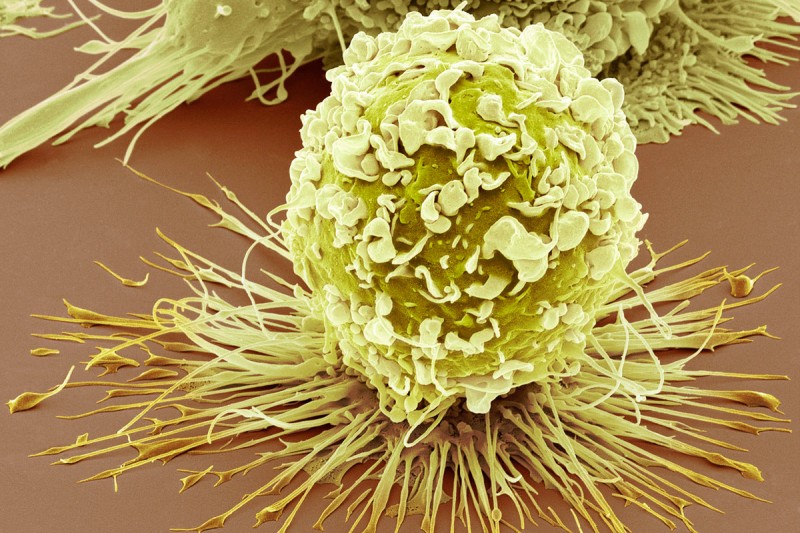
This scanning electron microscopy image shows an infection-fighting cell called a macrophage. Some macrophages that are located in tumors appear to help cancers grow. (Credit: Steve Gscheissner/Science Photo Library)
Memorial Sloan Kettering researchers have uncovered the origins of a mysterious population of immune cells living within tumors and discovered how the tumors manipulate these cells to their own advantage.
The possibility of harnessing a patient’s own immune system to attack tumors was first proposed more than 100 years ago but has only very recently become a reality. Striking clinical trial results have shown that drugs that unleash the immune system’s T cells can successfully treat several different types of cancer, including late-stage metastatic melanoma. This and other successes prompted Science magazine to name cancer immunotherapy the top Breakthrough of the Year in 2013.
Now the push is on to discover novel immunotherapies that are even more effective. But the relationship between tumors and the immune system is complicated. Many different types of immune cells are found within tumors. For example, some types of T cells have the potential to attack tumors, while other immune cell populations seem to help cancers grow. Immunologist Ming Li at Memorial Sloan Kettering is investigating how the immune system responds to tumors and how the different immune cells that colonize solid tumors interact to influence cancer’s growth and spread.
Subverted Defense
In a new study published in Science, Dr. Li’s team zeroed in on immune cells called macrophages that are found scattered throughout solid tumors. In healthy tissues, macrophages are an essential first-line defense against infection, but tumor-associated macrophages (TAMs) appear to help cancers grow.
“Although several clinical studies show that patients with higher numbers of tumor-associated macrophages have a poorer prognosis, we do not know where these cells come from, what exactly they might be doing to promote tumor progression, or how to stop them,” explains Dr. Li.
Using a mouse model of breast cancer, his team explored the origins and molecular makeup of TAMs. They discovered that TAMs develop from white blood cells that migrate into tumors as they begin to grow and then proliferate there. The development of TAMs depended on the activation of a molecular signaling pathway called Notch.
When the researchers knocked out a critical gene in this signaling pathway, the development of TAMs was inhibited, while the number of T cells within the tumors capable of killing cancer cells increased and tumor growth was suppressed. They propose that TAMs may aid tumor growth by turning off cancer-killing T cells that enter the tumor.
“Based on what we know about conventional macrophages, we were surprised to find that TAMs divide and rely on Notch,” says first author and graduate student Ruth Franklin. “Our findings indicate that TAMs are very different to the nondividing macrophages that populate healthy breast tissue and suggest ways to specifically target these cells for tumor immunotherapy.”
The team is now testing whether these findings hold true for human TAMs. If so, immunotherapies that inhibit TAMs could be a powerful approach to unleash tumor-attacking T cells, particularly if used in combination with other T cell-directed immunotherapies.
Benefits Beyond Cancer
Because the molecular fingerprint of TAMs is so different from that of macrophages that live in healthy tissues, Dr. Li suspects these cells represent a new type of immune response that may be important in other diseases.
Although macrophages that suppress T cells are bad news in cancer, in some situations they could be beneficial. For example, during chronic infections, excessive T cell activity can cause collateral damage to the body’s healthy tissues. Recruitment and proliferation of T cell–suppressing macrophages within tissues may have evolved as a way of protecting tissues from the immune system.
As tumors grow, they may in turn evolve ways to recruit and co-opt these specialized macrophages in order to ward off tumor-attacking T cells. To test this theory, the group is now exploring whether TAM-like cells arise during infections and in other disease settings.

