
From left to right: Joan Massagué, Director, Sloan Kettering Institute and Provost of the Louis V. Gerstner, Jr. Graduate School of Biomedical Sciences; GSK graduates Robert Bowman, Leili Ran, Isabel Lam, Elizabeth Wasmuth, Yilong Zou, and Lei Wei; Louis V. Gerstner, Jr., Chairman of the Board of Trustees, GSK.; GSK graduates Oakley Olson, Lindsay LaFave, Theresa Geiger, Chong Luo, and Cindy Puente; Kenneth J. Marians, Dean of the Louis V. Gerstner Jr. Graduate School of Biomedical Sciences, Chair of the Molecular Biology Program, and Director of Graduate Studies. (Not pictured: GSK graduate Armine Matevossian)
Twelve students at the Gerstner Sloan Kettering Graduate School of Biomedical Sciences have successfully defended their dissertations. As part of Memorial Sloan Kettering’s 37th annual academic convocation on May 19, the Louis V. Gerstner, Jr. Graduate School of Biomedical Sciences awarded PhD degrees during its fifth Commencement ceremony to Robert Bowman, Theresa Geiger, Isabel Lam, Lindsay LaFave, Chong Luo, Armine Matevossian, Oakley Olson, Cindy Puente, Leili Ran, Elizabeth Wasmuth, Lei Wei, and Yilong Zou.
The graduation of these students brings the number of graduates of the school to 37.

Robert Bowman completed his research in the laboratory of Johanna Joyce, focused on the tumor microenvironment of glioblastoma multiforme (GBM), a particularly aggressive form of brain cancer.
While the genetic mutations underlying GBM have been recently identified, comparatively less is known about the cells that make up the tumor microenvironment. In addition, the brain has long been considered an immune privileged site posing unique challenges in understanding the role of immune cells in this site.
And even though previous work has identified tumor-associated macrophages (TAMs) as therapeutic target in GBM, it remains unclear if these cells are derived from brain-resident macrophages (microglia) or monocyte-derived, peripheral macrophages. This is a particularly challenging question, as at the onset of Robert’s thesis, there were no tools to distinguish these two cells types. In his thesis he utilized multiple lineage tracing models and mouse models of glioma to interrogate this question. The results demonstrated for the first time that TAMs are composed of both brain-resident microglia and peripherally derived macrophages. Gene expression analyses revealed distinct transcriptional profiles for these cells reflective of their different developmental origins. These analyses identified a series of markers capable of distinguishing these cells.
He next used these markers in comprehensive flow cytometry panels to characterize the immune infiltrate in low-grade and high-grade glioma, as well as brain metastases in human patient samples. These studies have offered further insights into how developmental origins influence cellular response to inflammatory stimuli. Combined with the immune profiling on human tumor specimens, they lay the foundation to identify microenvironment-centric therapeutic avenues and markers of clinical response.

Theresa Geiger completed her dissertation research in the laboratory of Joseph Sun.
Theresa’s work focused on the development of innate lymphoid cells (ILCs). ILCs are a recently defined branch of the immune system and are important for protection from pathogens at barrier surfaces such as the intestine, lung, and skin.
The ILC family of cells includes group 1, group 2, and group 3 ILCs, which provide protection against viruses, parasites, and bacteria, respectively. Theresa’s work has shown that the transcription factor Nfil3, previously thought to be required for only group 1 ILC development, is critical for the development of all ILCs. She reported that loss of Nfil3 in mice results in a near complete loss of all types of ILCs, which renders the mice susceptible to intestinal pathogens. This work demonstrates that all ILCs share an early developmental relationship and identifies Nfil3 as a critical factor in promoting these important cells.

Working in the laboratory of Scott Keeney, Isabel Lam focused her thesis research on the meiotic recombination initiation landscape in yeast — its evolutionary dynamics and factors that shape its distribution.
Meiosis is a specialized cell division that reduces chromosome content in half and generates gametes — egg and sperm — in multicellular organisms. Budding yeast also undergoes the same process during formation of spores. Failure of cells to segregate their chromosomes properly during meiosis results in aneuploid gametes. In order for the reduction in chromosome content to occur accurately, chromosomes undergo homologous recombination initiated by DNA double-strand breaks (DSBs).
These DNA lesions are programmed to occur throughout the genome during meiosis, and they tend to be localized within narrow regions called hotspots. Isabel’s research explored the evolutionary fate of meiotic recombination hotspots. Two models have provided contrasting views: one viewpoint proposed more than 25 years ago predicts conservation of DSB hotspots if they coincide with functional genomic elements, as is the case in yeast, but many theoretical studies since then have dismissed this idea, and assumed that hotspots are always evolutionarily transient. Her work examining the meiotic DSB landscape in different Saccharomyces species provides empirical observation that DSB hotspots can be conserved despite 15 million years of evolution, therefore providing new insights into recombination evolutionary dynamics.

Lindsay LaFave conducted her graduate research in the laboratory of Ross Levine, investigating the mechanisms by which BAP1 and ASXL1 loss contribute to disease transformation utilizing both in vitro and in vivo loss-of-function model systems. She demonstrated that Bap1-null cells have an increased dependency on EZH2, a mechanism that can be targeted with small molecule EZH2 inhibitors.
Somatic mutations in epigenetic modifiers have recently been identified in hematopoietic malignancies and in other human cancers. However, the mechanisms by which these epigenetic mutations lead to changes in gene expression and disease transformation have not yet been well delineated. Lindsay’s work focused on studying epigenetic modifiers altered in human cancers, specifically chromatin regulators that modify post-translational modifications on histone molecules.
Mutations in the chromatin regulatorsASXL1 (Addition of sex combs-like 1) and BAP1(BRCA1 associated protein-1) have been identified in distinct diseases, with ASXL1 alterations most commonly found in myeloid malignancies and BAP1 alterations most commonly found in solid tumors (such as malignant mesothelioma). Using loss-of-function models (both in vitro and murine), Lindsay demonstrated that loss of ASXL1 and BAP1 have opposing effects on histone H3 lysine 27 trimethylation (H3K27me3), a repressive mark placed on chromatin by the Polycomb Repressive 2 complex, even though BAP1 and ASXL1 have been shown to interact in biochemical studies. Loss of BAP1 in the hematopoietic system resulted in increased H3K27me3 levels and decreased target gene expression, while the reverse was true for ASXL1.
Lindsay therefore proposed that BAP1 and ASXL1 had independent cellular activities significant for transformation. Importantly, mesothelioma BAP1-mutant and hematopoietic Bap1-null models were sensitive to both genetic knockout and pharmacological inhibition of EZH2 (Enhancer of Zeste homolog 2), the catalytic component that places the H3K27me3 mark. These preclinical data suggest that diseases with BAP1 alterations may be sensitive to targeted EZH2 inhibition.
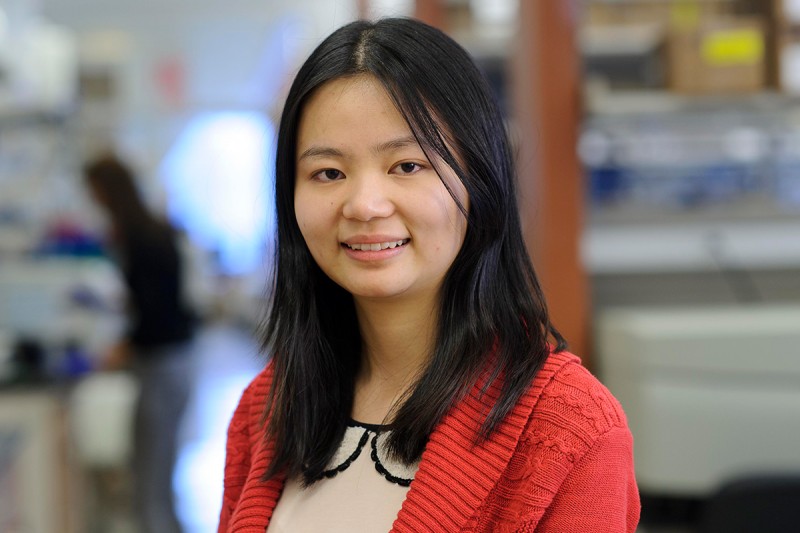
Working in the laboratory of Ming Li, Chong Luo focused her dissertation research on regulatory T cells (Tregs), which represent a suppressive T cell population that serves as a “brake” for immune responses. They are crucial in preventing the immune system from attacking healthy self-tissues and commensal microorganisms, but excessive Treg activities suppress anti-tumor immune responses.
Tregs in different anatomic locations are distinct — those in secondary lymphoid organs show a resting phenotype (rTreg), whereas those residing in non-lymphoid tissues, such as the liver, colon and tumor, exhibit an activated phenotype (aTreg). The study by Chong and her colleagues showed that aTregs had a slower turnover rate than rTregs, and were associated with heightened Akt signaling.
Foxo1 is a transcription factor that is negatively regulated by the PI3K/Akt signaling pathway, thereby aTregs contained a repressed Foxo1-dependent transcriptional program. Using a genetically modified mouse model in which an Akt-insensitive mutant of Foxo1 (a/k/a constitutively active Foxo1, or Foxo1CA) was expressed in Tregs, the authors revealed that aTreg homing to non-lymphoid organs were impeded while rTregs were minimally impaired, leading to a fatal autoimmune disease mediated by CD8+ T cells. In addition, Chong and her co-authors found that aTregs infiltrating the tumors down-regulated Foxo1-dependent program more substantially than those in the healthy tissues, suggesting that they might be more sensitive to the gain-of-function of Foxo1. Indeed, expressing Foxo1CA at a lower dose was sufficient to deplete tumor-associated Tregs, boost CD8+ T cell responses and inhibit tumor growth. Most intriguingly, expression of Foxo1CA at this dose did not trigger overt autoimmune responses. Therefore, this study provided a molecular foundation showing that the PI3K/Akt/Foxo1 pathway in Tregs can be titrated to selectively break immune tolerance in the tumor.
This research is of particular interest because high numbers of Tregs are found in tumors and are associated with poor prognosis in cancer patients. Many efforts have been made trying to augment anti-tumor immunity by eliminating Tregs. However, most therapies rely on broadly acting and non-specific medications, causing systemic depletion of Tregs and unrestrained autoimmune side effects. The work by Chong and her colleagues demonstrates that there is a potential therapeutic window that permits specific targeting of tumor-infiltrating Tregs.

Her thesis project focused on advancing our understanding of the structure and function of Hedgehog acyltransferase (Hhat), a transmembrane enzyme that mediates the attachment of a fatty acid onto the Hedgehog family of signaling molecules.
For one project, she set out to determine the significance of Hhat expression in breast cancer. Armine showed that Hhat is a novel target for inhibition of estrogen receptor-positive, HER2 amplified, and tamoxifen resistant breast cancer cell growth. Moreover, Hhat regulated the proliferation of both Sonic Hedgehog responsive and non-responsive estrogen receptor-positive cells, suggesting a Sonic Hedgehog independent function for Hhat.
In addition, to enhance our understanding of Hhat structure and function, Armine also conducted a comprehensive analysis of its transmembrane topology. She employed biochemical assays to demonstrate that Hhat contains ten transmembrane domains and two re-entrant loops and defined the topology of the active site.

Cancers develop in a complex microenvironment, in which tumor cells ‘hijack’ non-cancerous stromal cells from the surrounding tissue and bone marrow. Oakley’s research focused on one type of bone marrow-derived cell in particular, the tumor-associated macrophage (TAM).
Macrophages play important roles in many biological processes, such as tissue repair and response to infection. Cancer cells, however, are particularly adept at reprogramming macrophages into TAMs, causing them to suppress the immune response and remodel the local environment to promote tumor growth. Recently, Oakley and his colleagues have also shown that TAMs play an important role in regulating the response to chemotherapy in breast cancer.
When a tumor is treated with chemotherapy, many cancer cells die and macrophages are recruited to clear out the dead cells. The viable cancer cells then reprogram the macrophages to support tumor growth. Furthermore, these TAMs can additionally protect cancer cells from chemotherapy-induced cell death. Oakley also demonstrated the ability of TAMs to suppress the activity of chemotherapeutic agents that target the process of cell division, known as mitosis. These drugs block the ability of tumor cells to successfully segregate their DNA and divide, locking them in a “mitotic arrest.” He and his colleagues found that co-culture of breast cancer tumor cells with macrophages reduces the duration of mitotic arrest, causing the cells to “slip” out of mitosis without dividing. Furthermore, macrophages promote the persistence and survival of these breast cancer cells following mitotic failure, underscoring the potential role of TAMs in suppressing response to chemotherapy in breast cancer patients. This research may ultimately lead to therapeutic strategies for enhancing the efficacy of existing FDA-approved drugs.

Under nutritional stress, cells break down their own internal components for energy. This process is called autophagy. Autophagy proceeds by the de novo formation of a vesicle that engulfs intracellular components. This vesicle, called the autophagosome, mediates degradation and recycling of internalized components.
The autophagic process can be broken down into several discernable steps: induction, formation and degradation. Cindy’s project focused on further characterizing the induction step. She uncovered how nutrient starvation regulates the induction of autophagy. This finding further elucidates the molecular mechanisms that impinge on autophagy and identifies a novel method to modulate autophagy for future therapeutic benefit.

Gastrointestinal stromal tumor (GIST) is the most common type of sarcoma with around 5000 cases each year in United States. GIST usually arises in stomach and small intestine, followed by colon and cecum. Malignant peripheral nerve sheath tumor (MPNST) is a rare but very aggressive type of sarcoma. About 50 percent of MPNST is associated with disease that affecting one in every 3000 newborns called neurofibromatosis I (NF1).
Using comprehensive experimental approaches with human cancer cell line and mouse model for GIST and MPNST, Leili and her colleagues identified an important role of an oncogenic protein called ETV1 for the pathogenesis of both diseases. Furthermore, they demonstrated an effective therapeutic strategy to target the ETV1 protein, and effectively inhibited GIST tumor growth in multiple preclinical models. Based on their work, a phase Ib/II clinical trial has been initiated at Memorial Sloan Kettering Cancer Center to treat GIST patients.

The RNA exosome is a large, donut-shaped assembly of at least 10 to 11 distinct proteins that is involved in many fundamental cellular processes, including, but not limited to, maintaining proper levels of RNA to prevent unwanted accumulation, trimming nascent RNAs to maturation, or finding RNAs that have mistakes and clearing them out. Elizabeth used structural, biochemical, and genetic techniques to elucidate how the core scaffold works to coordinate the multiple RNA degradation activities of the exosome, how these activities influence each other, and how various other protein cofactors affect the specificity of the processing versus degradation activities of the exosome.
Using x-ray crystallography, Elizabeth obtained two near-atomic resolution models of 10- and 12-component exosomes, similar to those found in the nucleus. These structures provide snapshots of the exosome, with RNA engaged in its active site, poised for degradation. The protein architecture and molecular contacts defined in the model provide new insights into how this complicated assembly functions.
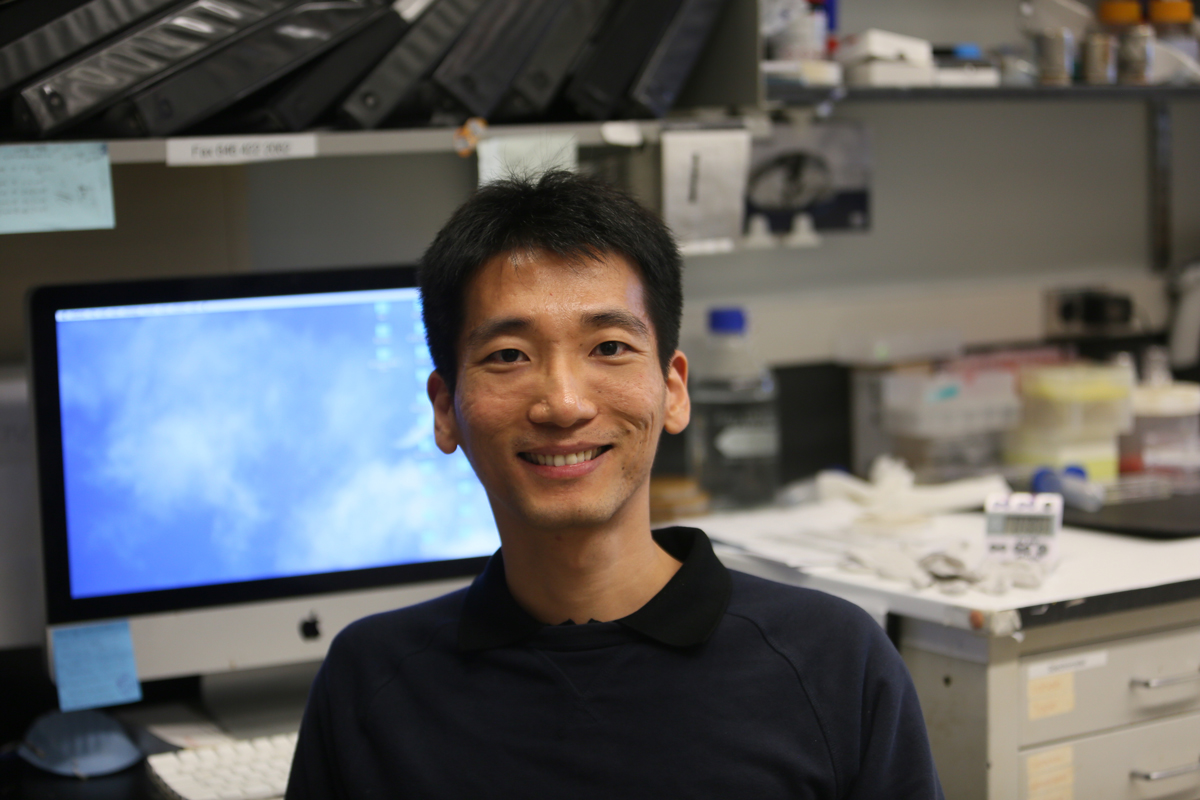
DNA replication and repair are two fundamental processes for the genome maintenance in all living organisms. These processes need to be regulated tightly so that they can only take place at the right time and in the right place. Dysregulated DNA replication and repair can lead to genome instability and numerous human diseases, including cancer.
In order to increase our understanding of the regulation of DNA replication and repair, Wei studied how three key proteins involved in these two processes are regulated. These three proteins are the DNA replicative helicase MCM, the DNA polymerase Pol2, and the single stranded DNA binding protein RPA. Using yeast genetic techniques, Wei made three novel findings: first, protein modification of MCM by SUMO ensures that DNA replication takes place at the right time; second, a part of Pol2 has a surprising function during replication; and third, the modification of RPA by SUMO has a novel function in DNA repair. These new findings provide critical missing pieces of how DNA replication and repair function.
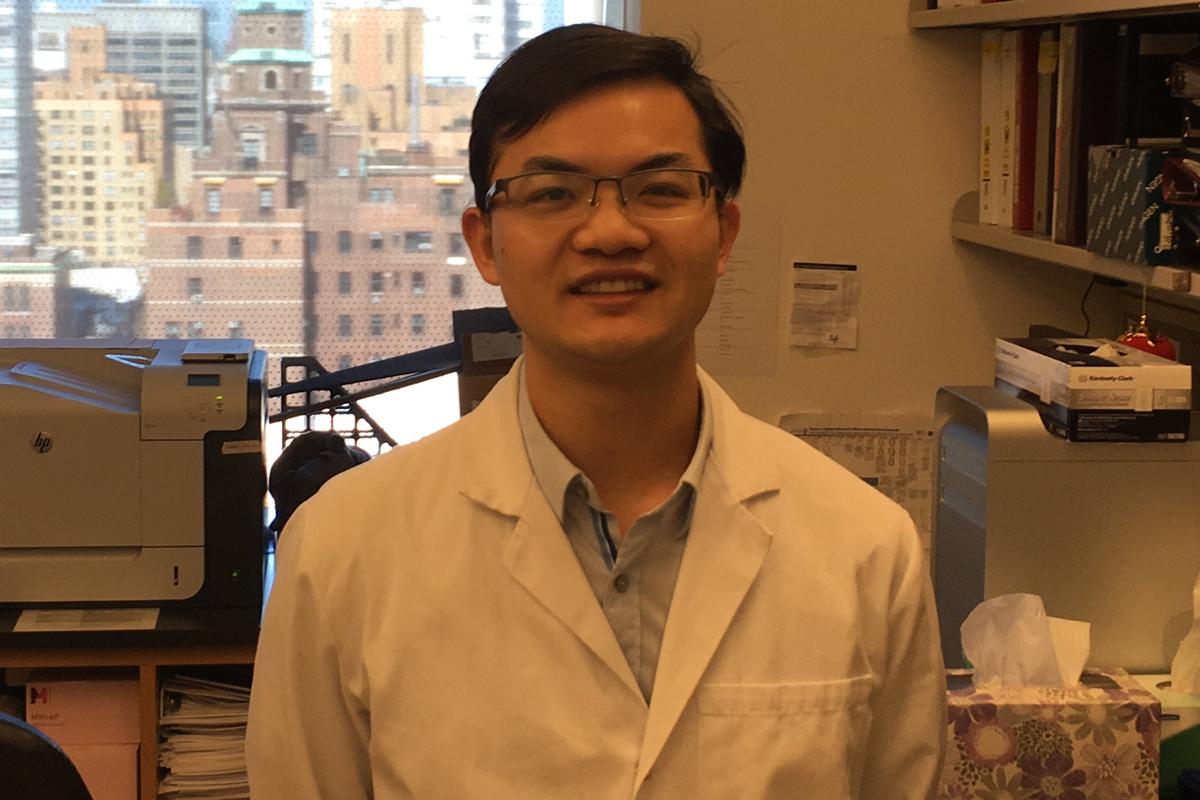
Pluripotent stem cells exhibit the potential to differentiate into every cell type in the human body and hold great promise for regenerative medicine. Yilong revealed that the p53 tumor suppressor family plays an important role in licensing the differentiation of pluripotent stem cells. His work demonstrated that the p53 family members directly drive Wnt expression to coordinate the Wnt-Tcf/β-Catenin and Nodal-Smad developmental pathways. This study provides a mechanistic basis for understanding the inhibitory role of p53 in induced pluripotency, and sheds light on the efficient induction and secure manipulations of pluripotent stem cells for cell therapy.
In addition, Yilong developed tools that allow the molecular characterization of metastatic stem cells in mouse models and assisted the identification of signaling pathways that are central to organ-specific metastasis development in multiple cancer types.