Due to the lack of effective screening and disease-specific symptoms, ovarian cancer is often diagnosed at an advanced stage, after malignant cells have already spread to the visceral and parietal peritoneum, resulting in peritoneal carcinomatosis characterized by widespread metastases of cancerous tumor deposits in the peritoneal cavity.
Upfront Surgery: Standard Treatment for Ovarian Cancer
The standard treatment for advanced ovarian cancer is a combination of surgery — with the goal to remove as much visible disease as possible — and platinum and taxane-based chemotherapy. However, the role of surgery and its risks and benefits have been continuously debated. Based on numerous studies and meta-analyses, superior survival outcomes have been observed in patients who undergo optimal surgery, with the goal to remove visible disease and to leave the patient with no detectable minimal residual disease before initiating chemotherapy. But surgery for advanced ovarian cancer is complex, often targeting multiple anatomic regions. As a result, surgical procedures have evolved to include en bloc gastrointestinal resections and upper abdominal procedures, targeting the liver, diaphragm, and chest. Beyond surgery, intraperitoneal (IP) chemotherapy has been shown to improve outcomes in patients with minimal residual disease after cytoreductive surgery. Both surgery and IP chemotherapy are associated with the potential for severe surgical complications and treatment-associated toxicity. Because of these challenges, complex cytoreductive surgery and IP treatment are often underused, and there is wide variability among centers in the performance of complex surgical procedures and the use of IP treatment.
Considering Neoadjuvant Chemotherapy
The delay of surgery has been the focus of recent efforts to decrease surgical morbidity. Two large, international, prospective, randomized clinical trials have evaluated the role of neoadjuvant chemotherapy (NACT) in patients with advanced ovarian carcinoma in an attempt to reduce tumor burden before performing surgery with intravenous (IV) chemotherapy, thus decreasing surgical morbidity. (1), (2) Both trials compared primary debulking surgery (PDS) followed by IV chemotherapy versus NACT followed by interval surgery and postoperative IV chemotherapy. Both studies showed significant decreases in surgical mortality and morbidity in patients who were administered NACT before surgery. Progression-free and overall survival were similar between the PDS and NACT arms in both trials. However, the median progression-free survival of ≤12 months and the median overall survival of ≤30 months in both studies were significantly less than those reported in other retrospective and prospective studies, which raises questions about patient selection and the quality of surgery received by patients randomized to the PDS study arms. This issue is evident in the low rates of optimal surgical cytoreduction in patients who had upfront surgery.
Upfront Surgery Versus NACT: The MSK Experience
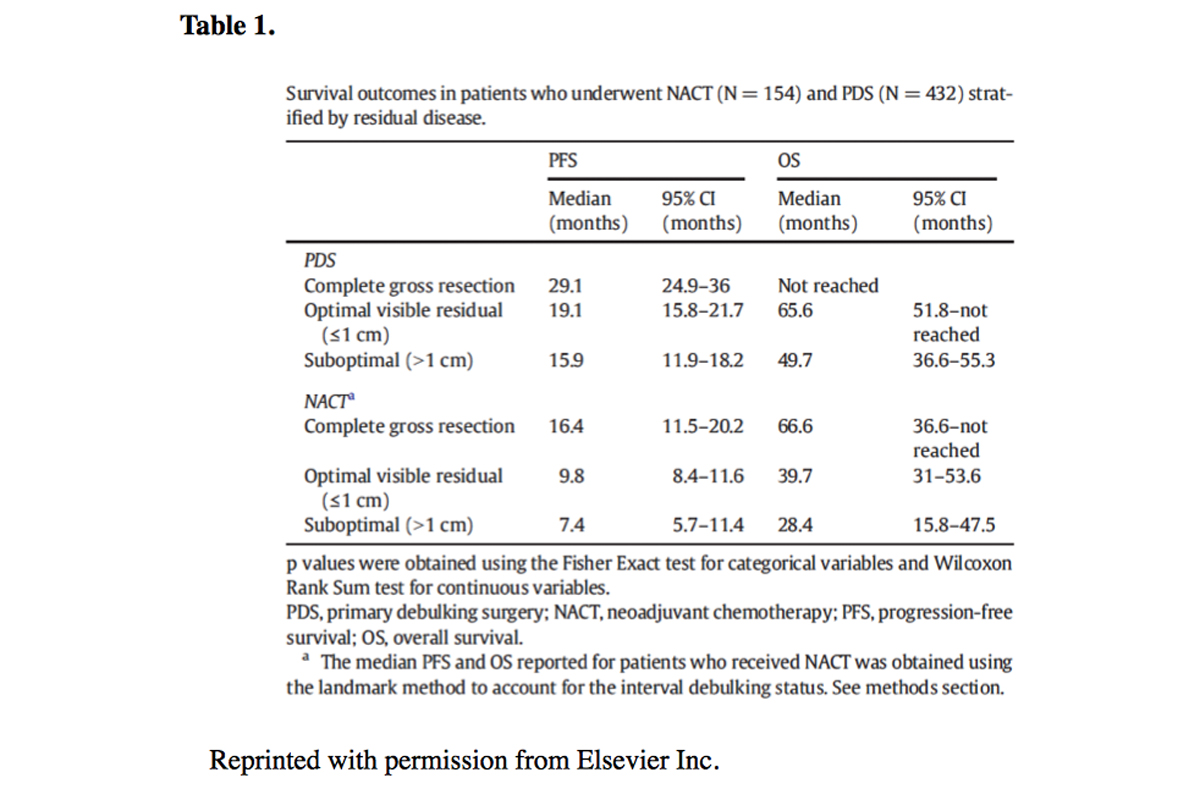
We recently analyzed the outcomes of patients with advanced, stage IIIC and IV, ovarian cancer treated at Memorial Sloan Kettering Cancer Center between 2008 and 2013. (3)Our study included 586 patients, with a median age of 62 years. A total of 154 women (26 percent) were treated with NACT and 432 (74 percent) received PDS. NACT use increased significantly from 22 percent before 2010 (when the results of one of the randomized trials was published) to 30 percent after 2010 and interestingly, no surgical mortality was seen. Although patients who underwent PDS were more likely to experience grade 3/4 surgical complications than those who underwent NACT, those selected for upfront surgery had a median overall survival of 72 months compared with 43 months for those selected for NACT. The median overall survival for all advanced-stage patients treated at our institution during the study period was 63 months, which is comparable to that of prospective studies that have included only optimally cytoreduced patients. (4) See Table 1.
Sticking With the Standard: Upfront Surgery
Our data show significantly higher optimal and complete gross resection rates, as well as significantly higher progression-free and overall survival, with PDS, contradicting the findings of published international trials, Our data also confirm a higher rate of surgical complications with upfront surgery, but not a higher rate of mortality. Therefore, we believe that the decision to proceed with NACT should be based on careful consideration of overall performance status, co-morbidities, the extent of disease, and patient preferences. While PDS is associated with an increased risk of surgical complications, it is also associated with the best outcomes. Balancing the risks of the procedure with patient preferences and oncologic outcomes should be carefully weighed when discussing final treatment strategy. While NACT is certainly the easier way to treat patients with advanced ovarian cancer, it may not be as effective as upfront surgery performed in high-volume surgical centers.
