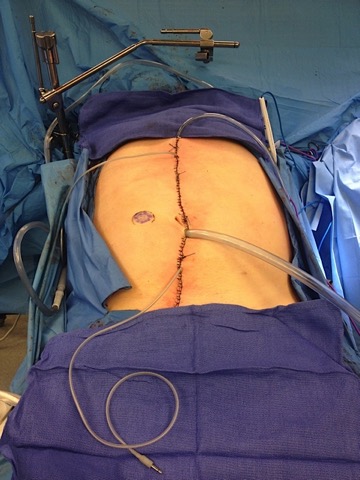
Figure 1. After surgical cytoreduction, percutaneous inflow and outflow catheters and IP temperature probes are placed in the peritoneal cavity. The catheters are connected to the perfusion system, and normal saline is heated to 41-43°C and circulated through the abdomen. When the desired intraperitoneal temperature is reached, the first dose of the chemotherapeutic agent (mitomycin) is added to the perfusate. The hyperthermic chemoperfusion is performed for 60 minutes, during which the perfusion rate, perfusion volume, and inflow and outflow temperatures are monitored and adjusted if necessary. A second dose of the chemotherapeutic agent is added, and 40 more minutes of chemoperfusion is performed. After completion of perfusion, the perfusate is drained and the abdomen is irrigated with normal saline.
Over the past 15 years, Memorial Sloan Kettering Cancer Center (MSK) and other specialty centers have been treating patients with peritoneal spread of appendix and colorectal cancer. Treatment includes debulking or cytoreductive surgery (CRS) to remove all visible tumor, followed by intraperitoneal chemotherapy (IPC), the delivery of chemotherapy directly into the abdomen to attempt to eliminate the microscopic disease that may remain after surgery.1 Using this approach, patients with appendix cancer have better long-term survival — some with more than 10 years — than some patients with other stage IV cancers.2 Colorectal cancer with peritoneal metastasis, however, is a more challenging problem; despite the most aggressive surgery and chemotherapy available, there are few cures.
Initial operations for this disease may be very long, up to 14 hours, although most are between five and ten hours. They may also be debilitating, typically requiring seven- to ten-day hospital stays. Our surgical goal is to remove all visible disease on the peritoneal surfaces, preserving the underlying organs whenever possible. However, it may be necessary to remove segments of the colon, small intestine, or stomach, as well as the gallbladder, spleen, or ovaries. Occasionally, an ileostomy or a colostomy is necessary, but usually only temporarily. Unfortunately, cancer recurs for 40 to 60 percent of patients, depending on the cancer subtype, requiring repeat operations.3
Given the high rates of recurrence and the large number of patients living with the disease, it is crucial that we gain a better understanding of optimal treatment. The histologic grade of the disease can help predict recurrence — low-grade tumors are less likely to recur than high- or intermediate-grade cancers — but it is still difficult to predict which patients will experience recurrence and when.
Two Intraperitoneal Chemotherapy Approaches for Appendix Cancer
Until now, no randomized trials have focused on the effectiveness of IPC for appendix cancer, and there has been only one completed trial of IPC for colon cancer. However, in several randomized trials of patients with ovarian cancer metastatic to abdominal surfaces, treatment with surgery and IPC has demonstrated efficacy for prolonging life — and, in select patients, even achieved a cure.
Two main types of IPC have been used in the United States: hyperthermic intraperitoneal chemotherapy (HIPEC) and early post-operative intraperitoneal chemotherapy (EPIC). Both are delivered directly into the abdomen to treat cancer where it is most likely to recur. HIPEC treatment consists of heated delivery of mitomycin into the abdomen through a catheter for 100 minutes after tumor removal surgery is completed (Figure 1). EPIC treatment involves delivery of floxuridine and leucovorin through an abdominal catheter for three days post-operatively, while the patient recovers in the hospital.
Non-randomized comparisons of patients undergoing HIPEC and EPIC have shown similar survival rates. However, these retrospective comparisons are inadequate to compare cancer outcomes definitively.
Clinical Trial: Intraperitoneal Chemotherapy After Cytoreductive Surgery (ICARuS)
At MSK, we have opened a randomized phase II clinical trial to compare HIPEC and EPIC in terms of effectiveness and toxicity: “Intraperitoneal Chemotherapy After cytoReductive Surgery” (ICARuS). We are also measuring the quality of life and restoration of nutrition in each treatment group and studying tumor genetics, correlating our findings with response or resistance to therapy. Hopefully, this will lead to targeted therapies for these types of cancers.
The ICARuS trial has been accruing very well. We have completed therapy in more than one-quarter of the planned 212 patients. To date, 80 percent of eligible patients have chosen to be included in the trial. Eligible patients are those who are considered able to undergo complete CRS for cancers of the appendix, colon, or rectum with isolated peritoneal metastasis. After CRS, patients in this study are randomly assigned to receive HIPEC or EPIC. Patients who have previously undergone IPC or have metastasis to sites other than the peritoneum — for example, the liver, lung, or bone — are not eligible.Surgical debulking has the potential to improve survival or palliate symptoms, but it may also result in significant complications, and quality of life is a primary concern for our patients. Therefore, we are excited to offer this unique trial. Our hope is that the results will give us a better understanding of optimal management of this disease for patients, patients’ families, and clinicians who confront daunting and difficult choices due to the uncertainty of the effectiveness of treatment options.
Patients interested in this trial can contact, or ask their physician to contact, Dr. Garrett Nash at 212-639-8668.
