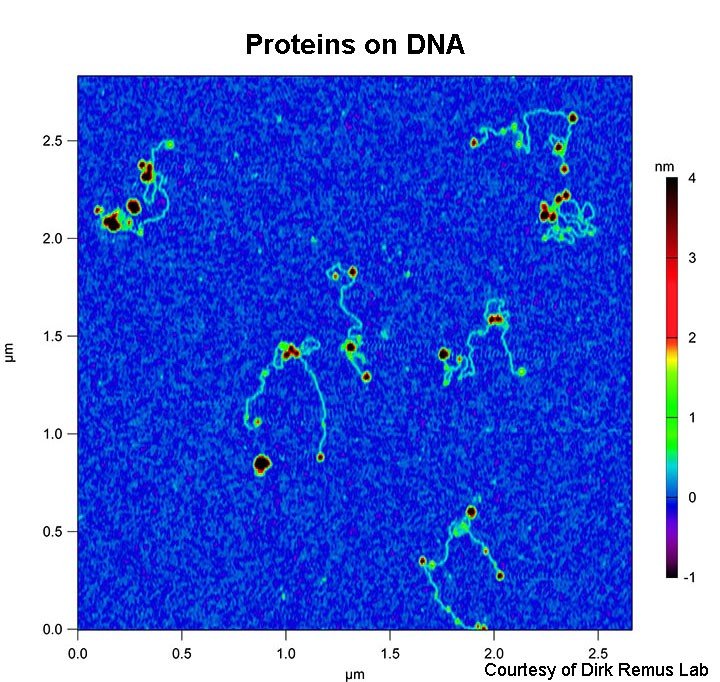
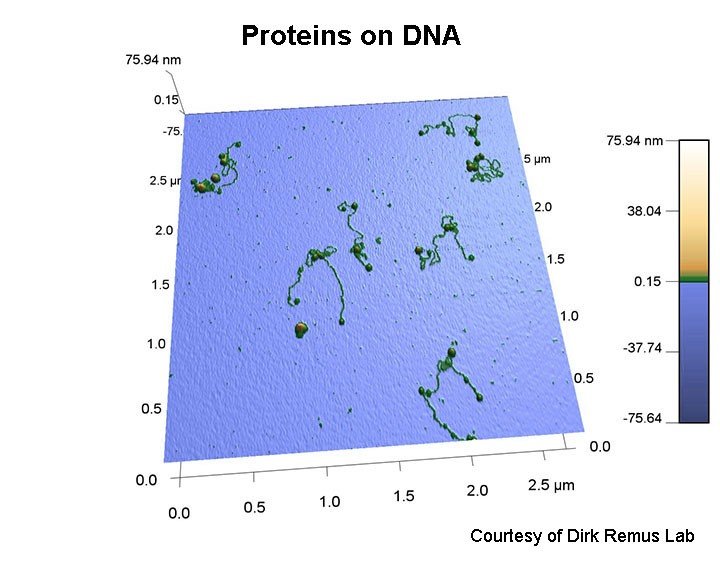
Objective: To expand the use of AFM for biological applications and enhance the methods available to scientists in the Tri-Institution and greater New York City area.
Asylum Research MFP-3D-BIO: Founded in 2013, the new AFM laboratory at Memorial Sloan Kettering’s Molecular Cytology Core Facility is fully equipped to handle a diverse range of AFM samples with the objective of expanding the use of AFM for biological applications. Our experienced operators have worked with more than 50 research labs on a diverse range of biological and non-biological samples. Our expertise is unparalleled, with imaging experience on well over 100 different samples. Our services include consultation on experimental design, operation of the microscope, and assistance with image analysis and data interpretation.
The AFM is an open and shared resource both within and outside MSK, and we welcome all scientists from the New York City area. Due to the complex nature of AFM experiments, we request that you contact us for initial consultations and planning of pilot experiments, which can be conducted within one to two weeks.
For information regarding scheduling and consultation, please contact Yevgeniy Romin at [email protected] or Biran Wang at [email protected], or call 646-888-2186.
Capabilities
All common imaging modalities: tapping mode, contact mode, force-indentation mode, force spectroscopy, phase imaging, frequency modulation, amplitude modulation, friction measurements, roughness measurements, et al.
Flexible environmental conditions: Imaging in air, imaging in fluid, fluid cell with temperature control, fluid cell with perfusion chamber, etc.
Examples of samples imaged or measured by the facility:
- Proteins
- Protein complexes
- DNA
- DNA-protein complexes
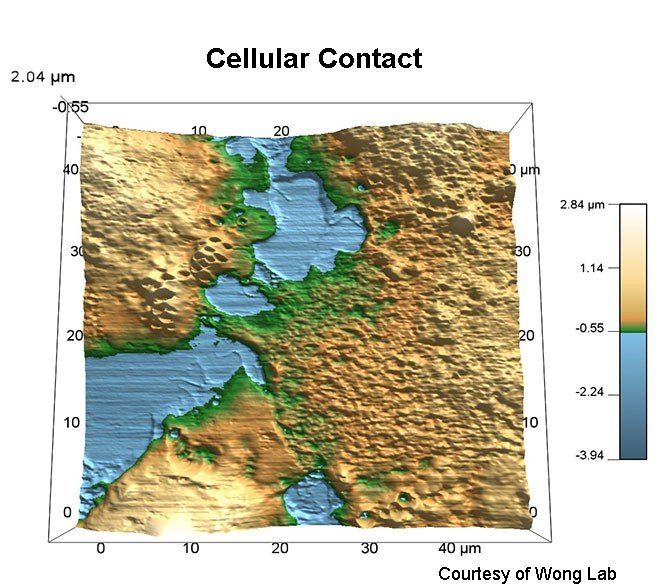
- Cells
- Cell membranes
- Tissues (liver, brain, nerve, kidney, bone marrow, bone, cartilage)
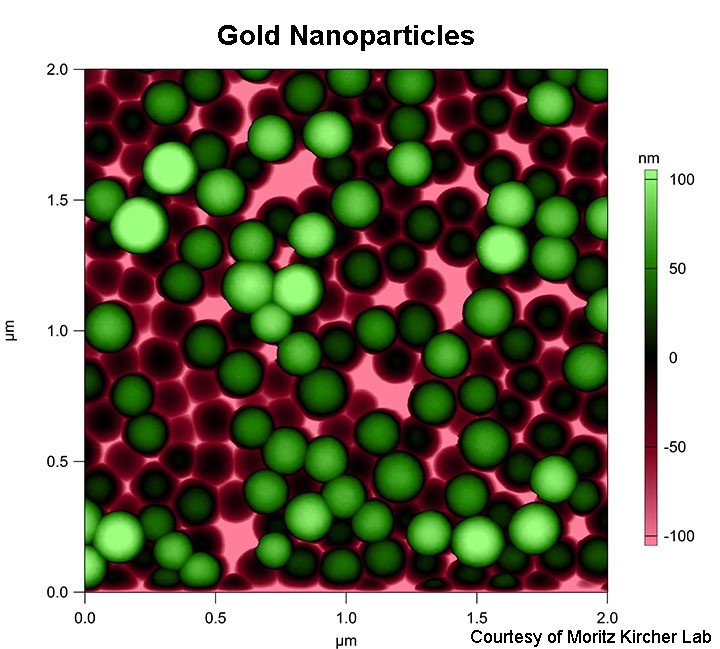
- Gold nanoparticles (as small as 10 nm, rods, spheres, stars)
- Silver nanoparticles (as small as 10 nm)
- Silica nanoparticles
- Single-walled carbon nanotubes (SWCNTs, CNTs)
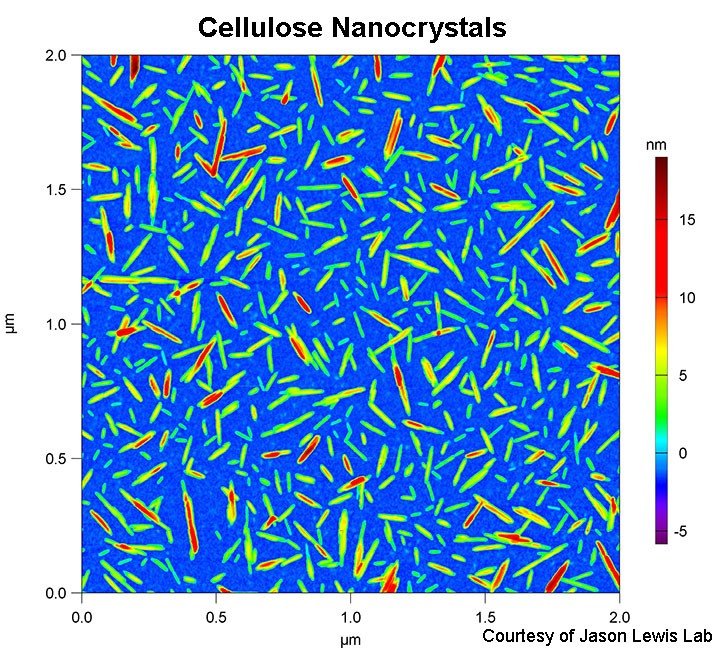
- Cellulose nanocrystals
- Feraheme
- Polymer nanoparticles
- Polymers
- Thin films
- Thin film lithography
- Supported lipid bilayers (SLBs)
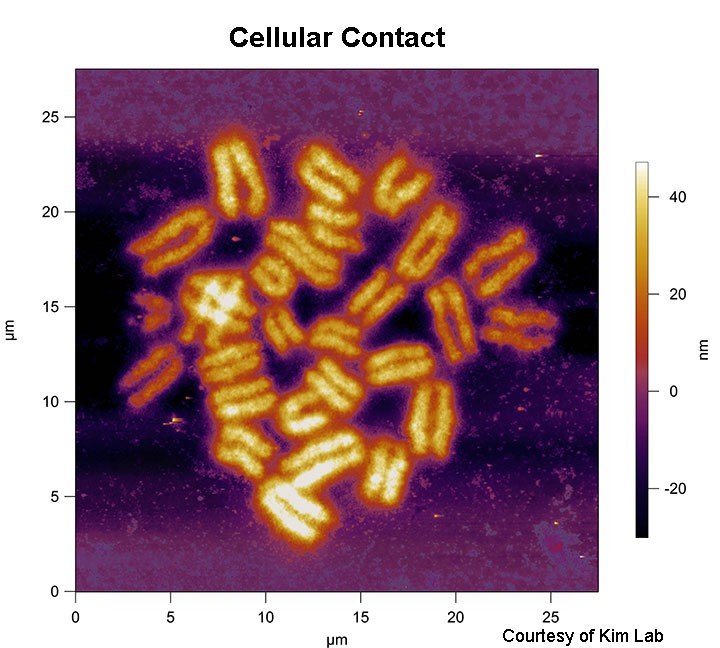
- Chromosomes and chromosome spreads
- Nuclei
- Exosomes and other lipid-bound vesicles
- Exosomal contents
- Amyloid-beta plaques (Ab42)
- Viruses
- Bacteriophage
- Crystals
- Plasmon-resonance surfaces
- Nanofiber meshes
- Tether pulling experiments
AFM+Inverted Optical Fluorescent Microscope: The AFM is mounted on a Zeiss Axio Observer A1 microscope, which can be used to combine AFM with optical techniques such as fluorescence imaging, brightfield imaging, phase, FRET, etc.
Diverse Probe Inventory: We keep a wide variety of probes in stock to facilitate faster experimentation. We stock all types of probes needed for all experiments listed above, with a selection of more than 100 models from various manufacturers.
AFM Limitations: The AFM is limited to imaging an X and Y range of 90 um, and a Z range of 40 um. Imaging speeds, in practice, are limited to 2 Hz. Fluid cell heating is from RT to 80 degC.