Over the past decade, we have used a combination of in vivo, in vitro, biochemical, and computational approaches to investigate various aspects of the biology of human cancer. Our ongoing efforts are aimed at addressing three broad biological questions:
- How do chromosomal rearrangements drive tumorigenesis and contribute to drug resistance?
- How do extrachromosomal circular DNAs (ecDNAs) contribute to tumorigenesis?
- How do non-coding RNAs regulate tumorigenesis, regeneration, and homeostasis?
In vivo and ex vivo chromosome engineering using the CRISPR-Cas9 system
Structural alterations of the genome are commonly observed in human cancer and can contribute to tumorigenesis through a variety of mechanisms including the formation of fusion oncoprotein and changes in copy number or expression of proto-oncogenes and tumor suppressor genes. Until recently, modeling these structural rearrangements in cells and in whole organisms remained technically challenging, time consuming, and costly.
The discovery of CRISPR-Cas9 and the development of somatic genome editing methods has profoundly changed the research landscape, providing unprecedented opportunities to faithfully recapitulate the complexity of the cancer genome in mice.
Our group has pioneered the use the CRISPR-Cas9 technology to engineer chromosomal rearrangements in vivo. In 2014 we described a simple strategy to engineer an oncogenic chromosomal inversion leading to the formation of an Eml4-Alk gene fusion in the lungs of adult wild type mice via intra-tracheal delivery of recombinant adenoviruses expressing Sp-Cas9 and 2 gRNAs targeting the desired genomic breakpoints. We showed the this is sufficient to induce the formation of fully penetrant lung adenocarcinoma that are histologically and clinically analogous to human EML4-ALK+ lung cancers (Maddalo et al., Nature 2014).
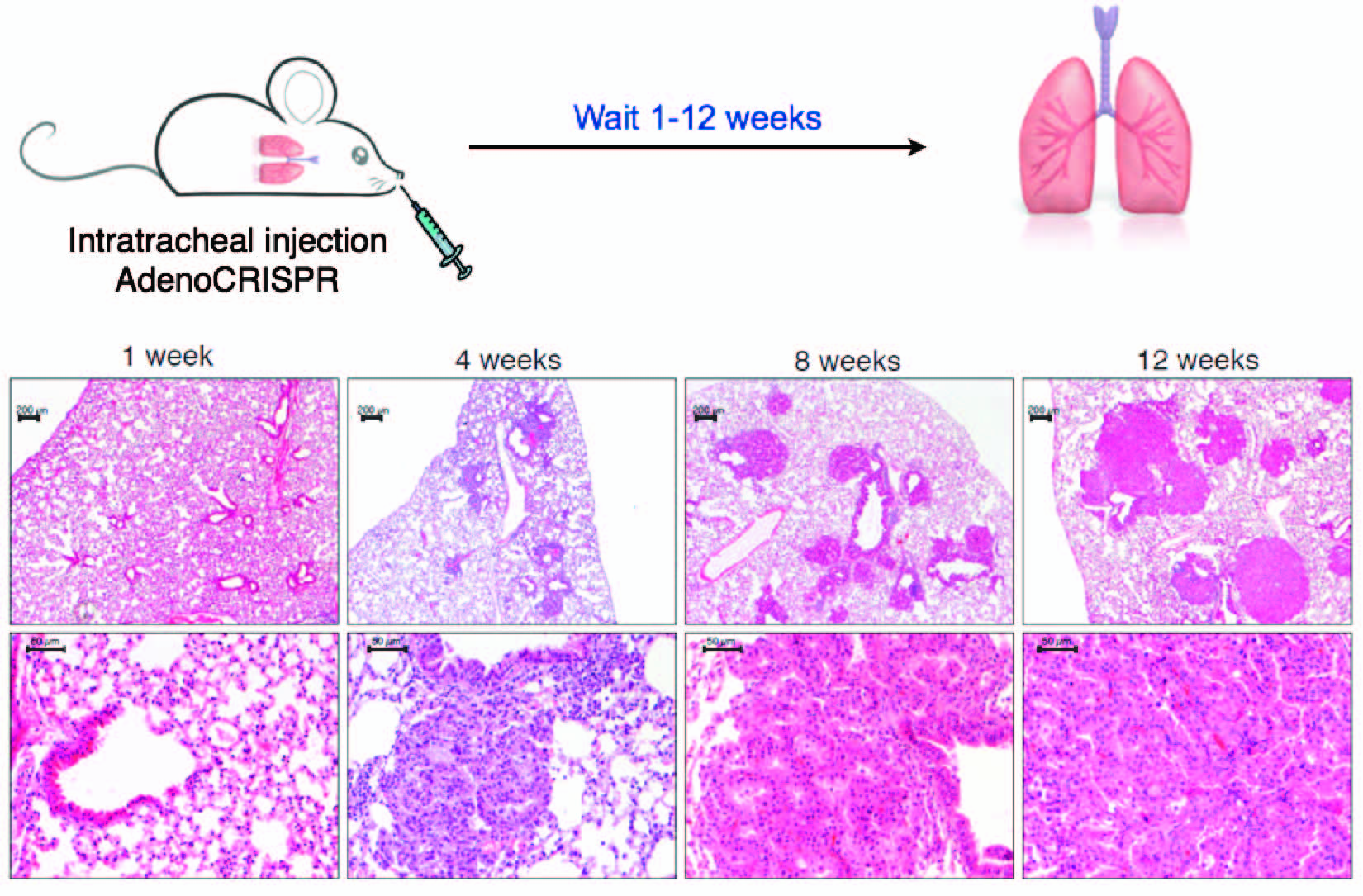
Histology of lung adenocarcinomas developing upon somatic induction of the Eml4-Alk inversion
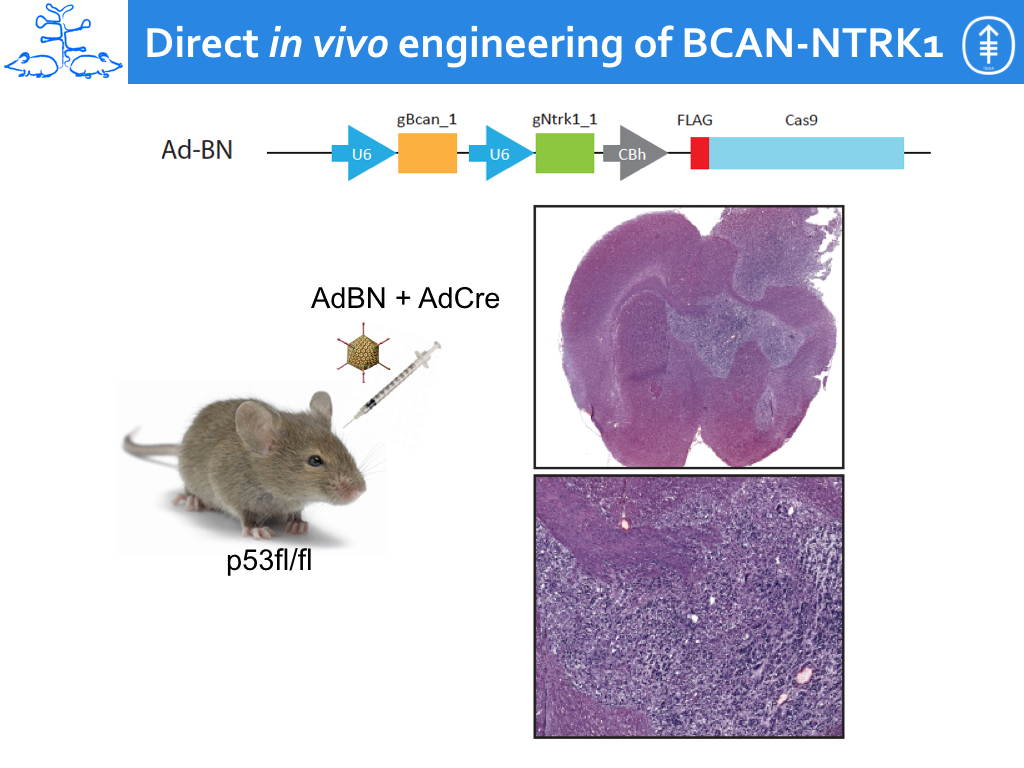
We have further applied this technology to show that a rare intrachromosomal deletion resulting in the formation of a BCAN-NTRK1 can drive the formation of highly aggressive gliomas in mice (Cook et al., Nature Communication, 2017).
To further facilitate the application of CRISPR-Cas9 technology in vitro and in vivo, in collaboration with the Leslie lab, we have recently developed new approaches to generate paired gRNA libraries (Vidigal et al., Nature Comm 2015) and a new computational platform for the rational design of highly specific guideRNAs (www.guidescan.com, Perez et al., Nature Biotechnology, 2017).
Ongoing projects are aimed at modeling and investigating pediatric brain tumors driven by a tandem duplication involving the BRAF oncogene and an aggressive pediatric sarcoma driven by a recurrent chromosomal translocation involving the WT1 and the EWS genes.
This work provides a general CRISPR-based strategy to model and characterize a wide range of chromosomal rearrangements found in human cancers, from simple deletions to translocations, inversions, and tandem duplication (Dow and Ventura, Annual Review of Cancer Biology, 2018).
ecDNA dynamics in cancer
Although known for half a century as “double minutes”, extrachromosomal circular DNAs (ecDNAs) have been recently the subject as intense investigation given their ability to harbor oncogenes and increase intratumoral heterogeneity. Despite their likely importance in human cancers, many questions about the genesis, evolution, and propagation of ecDNAs, as well as their contribution to tumor initiation and tumor progression remain unanswered
To answer these important questions, we have recently launched a major effort to develop a general strategy to engineer the formation of specific ecDNAs in cells and in whole animals and to follow their evolution using a range of imaging methods. Postdoctoral and pre-doctoral positions are available immediately to join our team and contribute to these efforts.
Biological functions of non-coding RNAs
Our work over the past decade has contributed to the detailed genetic and phenotypic characterization of the miR-17~92 and miR-34 families of miRNAs (Ventura et al., Cell 2008; Han, Vidigal, Mu et al., Nature Genetics, 2015, Concepcion et al., PLoS Genetics 2012). We have dissected the oncogenic function of miR-17~92 in lymphomas and prostate cancer (Mu et al., Genes & Dev., 2008) and we have identified, in collaboration with Jeanne Amiel’s group in Paris, hemizygous germline deletions of miR-17~92 as a common genetic cause of Feingold Syndrome, a developmental syndrome characterized by learning disabilities and skeletal defects (De Pontual, Yao, et al., Nature Genetics 2011).
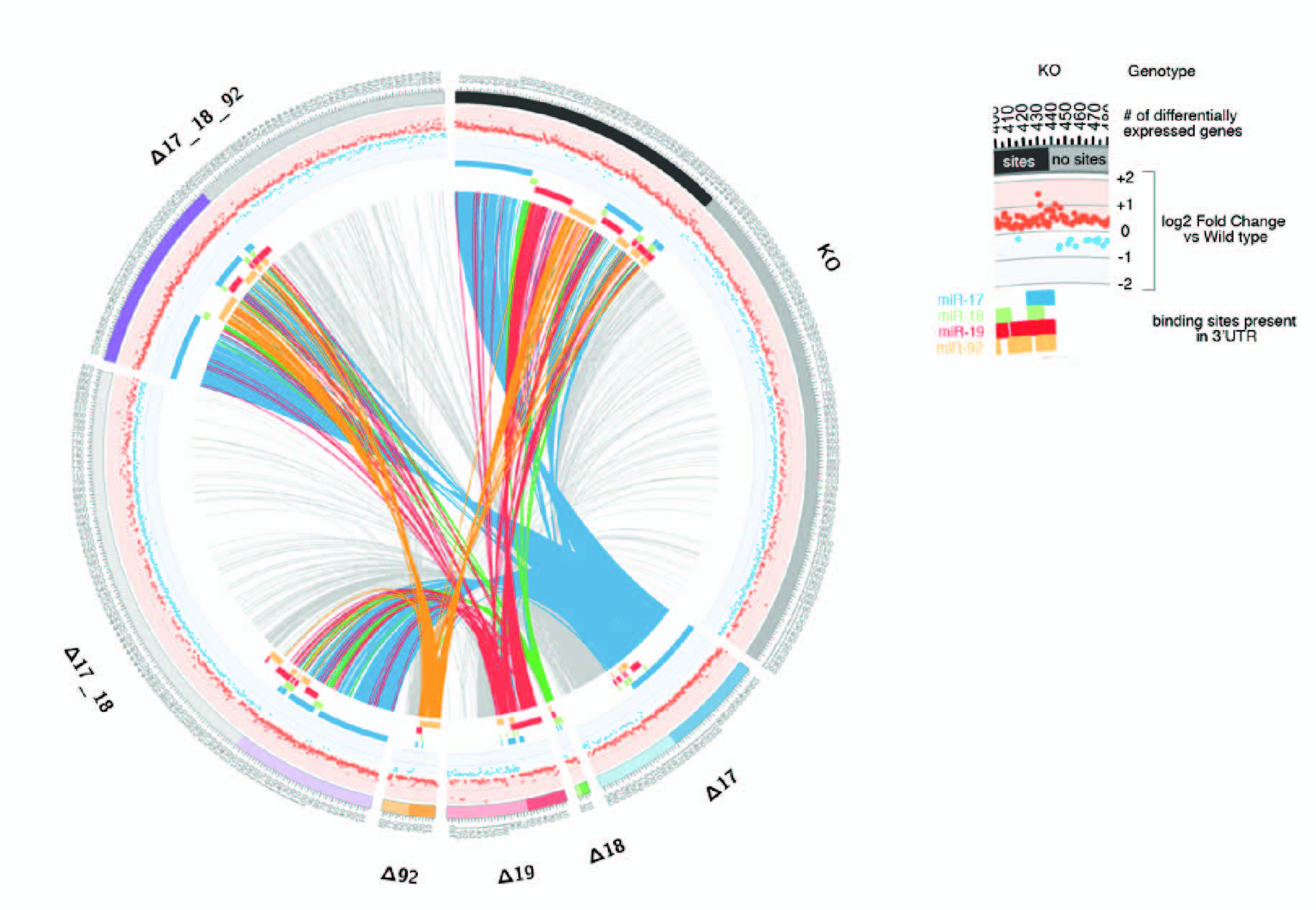
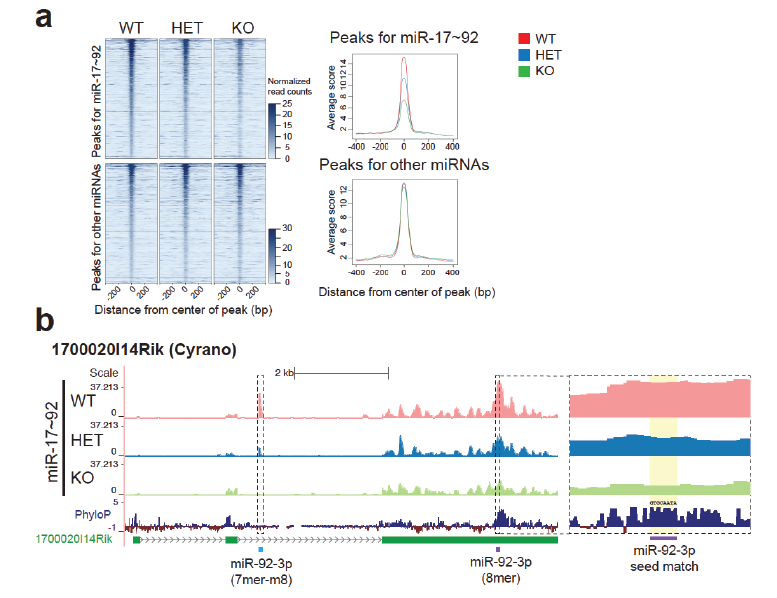
We have also been active in developing better tools to investigate miRNA functions. These include a novel method—Halo Enhanced Ago2 pulldown (HEAP)—based on a genetically engineered mouse strain that allows the direct identification of miRNA/mRNA interaction sites in cells and tissues (Li, Prytikin, Concepcion et al. Mol Cell 2020).
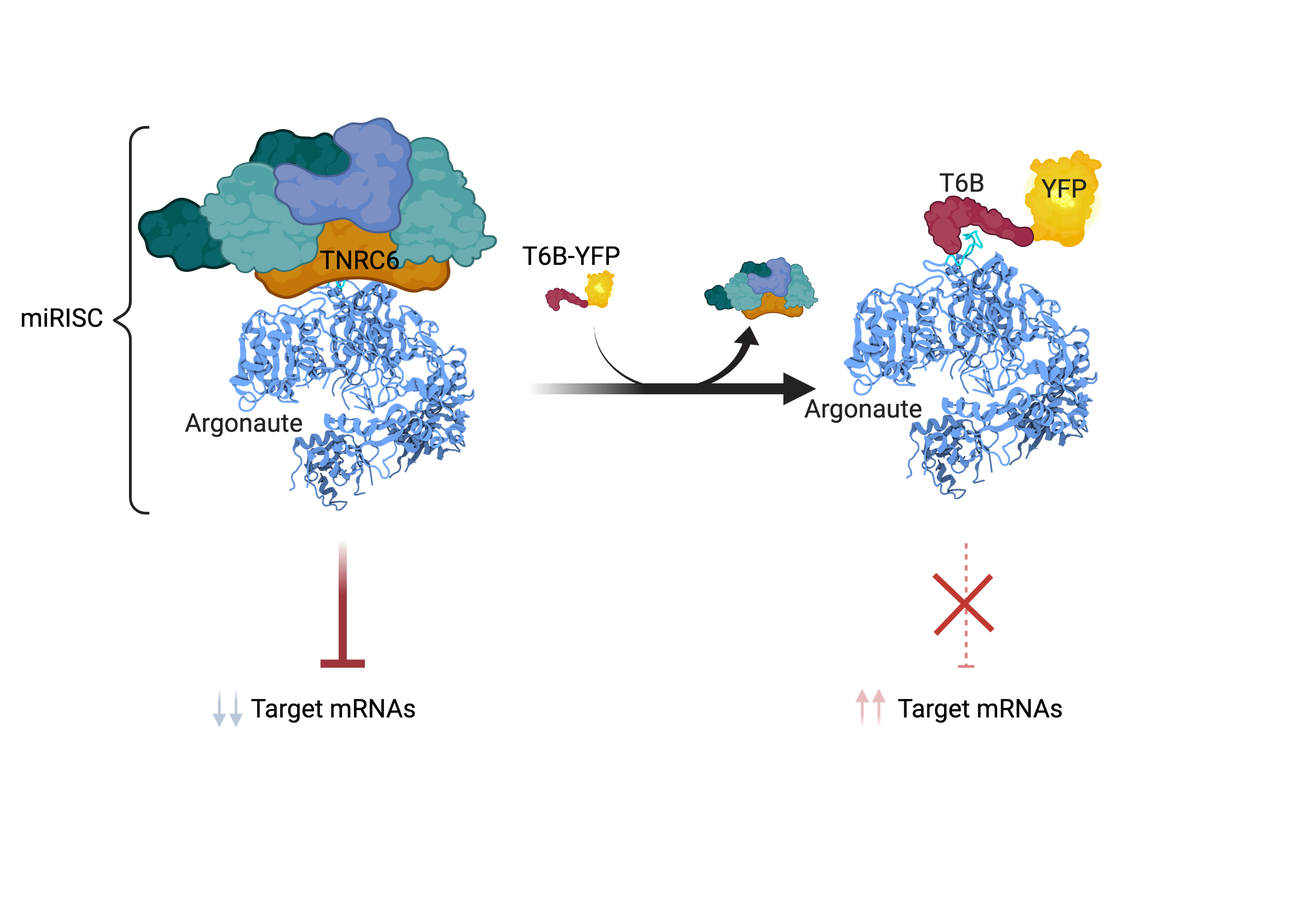
A major challenge in defining the biological functions of miRNAs in adult tissues has been the lack of an effective way to reversibly inhibit their function in a specific, temporally and spatially regulated, fashion. To address this limitation, we have recently generated a novel genetically engineered mouse model harboring a doxycycline-inducible transgene encoding a T6B-YFP fusion protein that, when expressed, prevents the formation of a functional miRISC complex thus acutely blocking miRNA-function. Using this mouse strain, we have shown that although miRNA-mediated gene repression is essential in the skeletal muscle and in the heart, for reasons that we currently do not understand, it is largely dispensable in many other organs and tissues of adult mice under homeostatic conditions. Interestingly, even in these tissues, miRNAs become essential during tissue regeneration and in response to acute tissue damage (La Rocca, King, et al., eLife 2021).
If you want to join our efforts to understand and defeat cancer, apply to one of the available positions in our lab. For more information on our current projects, please follow this link.