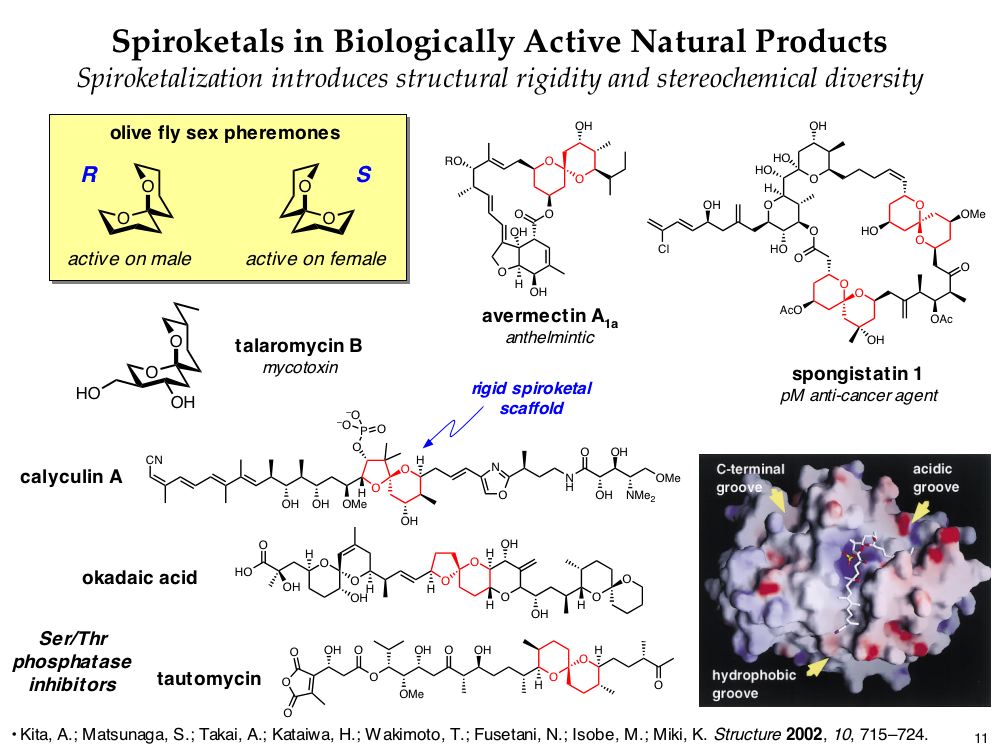
Spiroketal Motifs in Biologically Active Natural Products
Spiroketals are privileged substructures found in numerous natural products. Even relatively simple spiroketals exhibit a range of biological activities. In more complex structures, spiroketals often serve as rigid scaffolds to present sidechains along well-defined three-dimensional vectors. Thus, spiroketals are attractive targets for diversity-oriented synthesis. In particular, they allow extensive use of stereochemical diversity to enhance three-dimensional structural diversity.
Target-oriented synthesis of spiroketal-containing natural products has commonly relied upon thermodynamically-controlled spiroketalization reactions. However, in such reactions, the stereochemical outcome at the anomeric carbon is influenced by multiple, sometimes competing factors that govern product stability. Thus, to synthesize systematically stereodiversified spiroketal libraries, new kinetically-controlled spiroketal-forming reactions are required.
We have developed a new synthetic route to spiroketals that provides stereocontrolled access to either of the two anomeric stereoisomers. Our approach also uses four adjacent stereogenic centers to present functional groups and sidechains along diverse three-dimensional vectors. This project has also provided us with many exciting opportunities to develop new synthetic methodologies. Our current efforts involve applications of these methods to the diversity-oriented synthesis of spiroketal libraries and to the total synthesis of spiroketal natural products.
Publications
Family-level stereoselective synthesis and biological evaluation of pyrrolomorpholine spiroketal natural product antioxidants
Verano, A. L.; Tan, D. S.* Chem. Sci. 2017, 8, 3687–3693.
[ Abstract | PubMed | PMC ]-
Stereocontrolled Synthesis of Spiroketals: An Engine for Chemical and Biological Discovery
Verano, A. L.; Tan, D. S.* Isr. J. Chem. 2017, 57, 279–291.
[ Abstract ] -
Solvent-dependent divergent functions of Sc(OTf)3 in stereoselective epoxide-opening spiroketalizations.
Sharma, I.; Wurst, J. M.; Tan, D. S.* Org. Lett. 2014, 16, 2474–2477.
[ Abstract | PubMed | PMC ] -
Stereoselective synthesis of acortatarins A and B.
Wurst, J. M.; Verano, A. L.; Tan, D. S.* Org. Lett. 2012, 14, 4442–4445.
[ Abstract | PubMed | PMC ] -
Hydrogen-bonding catalysis and inhibition by simple solvents in the stereoselective kinetic epoxide-opening spirocyclization of glycal epoxides to form spiroketals.
Wurst, J. M.; Liu, G.; Tan, D. S.* J. Am. Chem. Soc. 2011, 133, 7916–7925.
[ Abstract | PubMed | PMC ] -
Stereoselective synthesis of benzannulated spiroketals: Influence of the aromatic ring on reactivity and conformation.
Liu, G.; Wurst, J. M.; Tan, D. S.* Org. Lett. 2009, 11, 3670–3673.
[ Abstract | PubMed | PMC ] -
Stereocontrolled synthesis of spiroketals via Ti(Oi-Pr)4-mediated kinetic spirocyclization of glycal epoxides with retention of configuration.
Moilanen, S. B.; Potuzak, J. S.; Tan, D. S.* J. Am. Chem. Soc. 2006, 128, 1792–1793.
[ Abstract | PubMed | PMC ]
(Highlighted in Nature ) -
Stereocontrolled synthesis of spiroketals via a remarkable methanol-induced kinetic spirocyclization reaction.
Potuzak, J. S.; Moilanen, S. B.; Tan, D. S.* J. Am. Chem. Soc. 2005, 127, 13796–13797.
[ Abstract | PubMed ] -
An acid-stable tert-butyldiarylsilyl (TBDAS) linker for solid-phase organic synthesis.
DiBlasi, C. M.; Macks, D. E.; Tan, D. S.* Org. Lett. 2005, 7, 1777–1780.
[ Abstract | PubMed ]
(Highlighted in Lett. Org. Chem. [PDF]) -
Enantioselective synthesis of erythro-4-deoxyglycals as scaffolds for target- and diversity-oriented synthesis: New insights into glycal reactivity.
Moilanen, S. B.; Tan, D. S.* Org. Biomol. Chem. 2005, 3, 798–803.
[ Abstract | PubMed ] -
Synthesis of C1-alkyl and C1-acylglycals from glycals using a B-alkyl Suzuki–Miyaura cross coupling approach.
Potuzak, J. S.; Tan, D. S.* Tetrahedron Lett. 2004, 45, 1797–1801.
[ Abstract ]
News Articles
02/02/2006
Chemistry: Order of the rings
Nature
One of the great challenges in organic chemistry is developing novel ways to synthesize complex structures. The best reactions work for a range of chemical substrates, and give high yields. By these criteria, a reaction, discovered by Derek Tan of the Memorial Sloan-Kettering Cancer Center in New York and his co-workers is near ideal. [Full text]