Investigation of antiviral innate immunity, viral immune evasion mechanims, and host defense against vaccinia infection
Poxviruses are large cytoplasmic DNA viruses that cause significant human and veterinary diseases. Vaccinia virus is a prototypical poxvirus successfully used for smallpox eradication. Oncolytic vaccinia viruses are promising cancer immunotherapeutics. Modified vaccinia virus Ankara (MVA), an attenuated vaccinia strain, is a safe and effective vaccine vector against various infectious agents and cancers. Our research team has uncovered several important poxvirus-sensing mechanisms leading to type I interferon (IFN) induction in various cell types including epithelial cells, macrophages and dendritic cell (DC) subtypes. Our publication in PLoS Pathogens (2014) demonstrated that infection of conventional DCs with MVA, leads to the induction of type I IFN production that is dependent on the cytosolic DNA sensor cGAS (cyclic GMP-AMP synthase) and its adaptor STING (stimulator of IFN genes). Since then, we have been focusing on identifying viral inhibitors of the cGAS/STING pathway through biochemical studies, as well as the generation and characterization of recombinant vaccinia viruses or MVA lacking viral inhibitors. The results of these work will have important implications for improving MVA as a vaccine vector and an immunotherapeutics agent. Skin acts as the first line of defense against invading microorganisms: not only does skin provide a physical barrier to pathogen entry, but it also initiates vigorous innate immune responses upon sensing danger signals. Upon invasion, viruses release or produce viral nucleic acids, which are potent stimulators for the host skin immune system that trigger antiviral innate immunity and inflammation. But how cutaneous immune system detects viruses and restricts their spread is not well understood. To that end, we showed how murine primary keratinocytes (KCs) can mount vigorous innate immune responses to cytosolic dsRNA, which can be antagonized by a vaccinia virulence factor E3. Both the cytosolic DNA and dsRNA-sensing pathways play non-redundant roles in host defense against vaccinia infection intranasally. We will continue to take an interdisciplinary approach to studying virus-host interactions. At the transcriptome level, we have been conducting RNA-seq analyses of skin keratinocytes, fibroblasts, and dendritic cells from WT mice and those deficient in DNA or RNA-sensors or adaptors with or without viral infection. We have identified various gene sets regulated by the nucleic acid-sensing mechanisms at a basal level and upon stimulation with pathway agonists. We aim to discover novel antiviral genes and positive and negative regulators of these pathways, and to assess their functions in vivo using viral infection models. The results of these work will illuminate the network of host defense mechanisms in the skin.
Developing poxvirus-based cancer immunotherapeutics
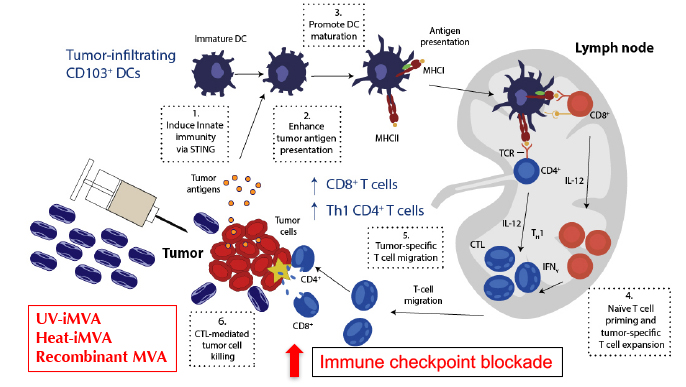
Despite successes in immunotherapy, the majority of patients fail to respond to immunotherapy alone; therefore, further investigations of mechanisms underlying resistance to immunotherapy and the development of novel combinatorial approaches are urgently needed. Understanding host innate immune sensing of poxviruses and viral immune evasion strategies would allow us to improve vaccinia-based immunotherapeutics and to design novel viral-based strategies to synergize with, or to overcome resistance, to immune checkpoint blockade therapy. Given the clinical need to incite an inflammatory response in “cold tumors” that do not respond to current checkpoint inhibitors, our goal is to develop safe and effective vaccinia-based cancer immunotherapeutics. Our recent publication in Science Immunology (2017) demonstrated that intratumoral (IT) injection of heat-inactivated MVA (Heat-iMVA; by heating MVA at 55˚C for 1 h) results in the regression of tumors at the injected sites and rejection of tumor challenge at distant site in murine tumor models. The combination of IT Heat-iMVA with systemic delivery of immune checkpoint blockade antibodies achieves enhanced antitumor efficacy. we will continue to develop two platforms of vaccinia-based immunotherapeutics: (i) recombinant MVA expressing immunomodulatory agents; and (ii) replication-competent attenuated oncolytic virus expressing immunomodulators including immune checkpoint blockade antibodies.
Poxvirus modulation of type I interferon (IFN) production
Over the past decades, major progress has been made toward understanding how viruses are recognized by the immune system to trigger antiviral immune responses and how viruses evade host defense mechanisms. Several viral-sensing molecules have been identified, including Toll-like receptors (TLRs), RIG-I like receptors (RLRs), and cytosolic DNA sensors. Both TLR-dependent and TLR-independent sensing mechanisms leading to interferon (IFN) induction in the host cells have been identified for host detection of poxviruses. We have reported that infection with attenuated vaccinia virus with deletion of virulence factor E3 (a Z-DNA/dsRNA binding protein) induces type I IFN production by epithelial cells via a cytosolic dsRNA sensing mechanism dependent on mitochondrial antiviral signaling protein (MAVS; an adaptor for RIG-I and MDA5) and the transcription factor IRF3. The C-terminal dsRNA binding domain of E3 plays an inhibitory role. We have recently reported that myxoma virus, a Leporipoxvirus that causes lethal myxomatosis in European rabbits, induces type I IFN production in plasmacytoid dendritic cells, via a TLR9/MyD88-, IRF5/IRF7-, and IFNAR-dependent pathway. This pathway is inhibited by the N-terminal Z-DNA binding domain of E3, which is missing in the ortholog M029 protein expressed by myxoma virus. In conventional dendritic cells, infection of the highly attenuated modified vaccinia virus Ankara (MVA), the current vaccine for smallpox, induces type I IFN production via an IRF3-dependent mechanism involving DNA sensing. This pathway is also inhibited by the Z-DNA binding domain of E3. Therefore, poxvirus infection is detected by various host innate antiviral sensing mechanisms in a cell-type-specific manner. Current studies focus on the identification of novel components of poxviral sensing pathways, mechanisms of viral immune evasion by vaccinia E3 and other viral immunomodulatory proteins, and the role of an innate immune sensing pathway in host defense against poxvirus infection.
Poxvirus modulation of autophagy
We have developed a new line of research on poxviral modulation of autophagy. Autophagy, or self-eating, is a cellular response to nutrient starvation or other environmental cues and stress involving degradation of its cellular components through the lysosomal apparatus. The hallmark of autophagy is the formation of autophagosomes and autolysosomes. Autophagy proceeds in several stages: initiation, vesicle nucleation, vesicle elongation and maturation, and vesicle fusion with late endosomes and lysosomes followed by degradation of the vesicle contents. The initiation of autophagy involves inhibition of TOR (target of rapamycin), leading to the activation of Atg1/ULK1. Vesicle nucleation occurs through the activation of Class III phosphatidylinositol 3-kinase (PI3K or Vps34) to generate phosphatidylinositol-3-phosphate (PIP3) via the formation of a multiprotein complex including Beclin-1 (mammalian orthologue of Atg6). Vesicle elongation requires two ubiquitin-like conjugation systems. One system involves the covalent conjugation of Atg12 to Atg5. The other system involves the conjugation of phosphatidylethanolamine (PE) to Atg8 (LC3 in mammals). Lipid conjugation leads to the conversion of the soluble form of LC3 (LC3-I) to the autophagic-vesicle-associated form (LC3-II). LC3-II is used as a marker for autophagy because its lipidation and specific recruitment to autophagosomes provide a shift from diffuse to punctate staining pattern under light microscopy and increase its electrophorectic mobility compared with LC3-I using western blot analysis.
Autophagy plays important roles in host antiviral innate and adaptive immune responses. Many viruses have evolved strategies to evade autophagy. How poxvirus modulates autophagy is unclear. We find that infection with attenuated vaccinia virus MVA or myxoma virus induces autophagy whereas infection with wild-type vaccinia does not. Autophagy induction is critically linked to host sensing of poxvirus infection and type I IFN induction. We are currently investigating the cross-talk between autophagy and DNA sensing pathways, the mechanisms mediating poxviral induction or inhibition of autophagy, and the role of autophagy machinery in host defense against poxvirus infection.
Poxvirus as oncolytic and immunotherapy for melanoma
Advanced melanoma is largely refractory to conventional therapies, including chemotherapy and radiation. The discovery of somatic mutations in the serine-threonine kinase BRAF in about 50 percent of melanomas opened the door for targeted therapy in this disease. Early clinical trials with BRAF inhibitors showed remarkable but not sustainable responses in patients with melanomas with BRAF mutation. Therefore, alternative treatment strategies for patients with wild-type BRAF and with BRAF inhibitor-resistant tumors are urgently needed.
Poxviruses hold promise as oncolytic and immunotherapeutic agents for cancers. Our goal is to develop attenuated vaccinia viruses for melanoma therapy. Our preliminary studies indicate the use of attenuated vaccinia viruses might provide a novel strategy to achieve melanoma cell killing and to initiate innate and adaptive immune responses against melanoma. We currently are investigating the mechanisms of induction of innate immune responses and apoptosis in melanoma cells by attenuated vaccinia virus ∆E3L in human and murine melanoma cells and the immunological mechanisms mediating tumor eradication through intratumoral injection of viruses in murine melanoma models. We also investigate the therapeutic efficacy of the combination of virotherapy and blockade of cytotoxic T lymphocyte-associated antigen 4 (CTLA-4), a key negative regulator of T cell activation, in animal models. Clinical trials of fully humanized anti-CTLA-4 monoclonal antibodies have shown impressive results with durable responses and improved survival in advanced melanoma patients, which led to the recent FDA approval for the treatment of unresectable or metastatic melanoma. We propose that infection of tumor cells with attenuated vaccinia results in tumor cell death, release of tumor antigens, and the recruitment of inflammatory cell infiltrates leading to the induction of tumor specific adaptive immunity, which is enhanced in the presence of CTLA-4 blockade.