-
Laser-scanning Confocal Microscopes
 Two Leica SP5 (Upright and Inverted Stands) - Leica SP5’s are point-scanning confocal microscopes capable of taking high resolution optical sections at high speed. The inverted stand can accommodate live samples and time-lapse experiments in addition to traditional slides. The AOBS on these systems allows users to conduct spectral separation in which autofluorescence and signals of similar spectra can be separated. The upright microscope also have an additional capability of conducting FLIM experiments.
Two Leica SP5 (Upright and Inverted Stands) - Leica SP5’s are point-scanning confocal microscopes capable of taking high resolution optical sections at high speed. The inverted stand can accommodate live samples and time-lapse experiments in addition to traditional slides. The AOBS on these systems allows users to conduct spectral separation in which autofluorescence and signals of similar spectra can be separated. The upright microscope also have an additional capability of conducting FLIM experiments.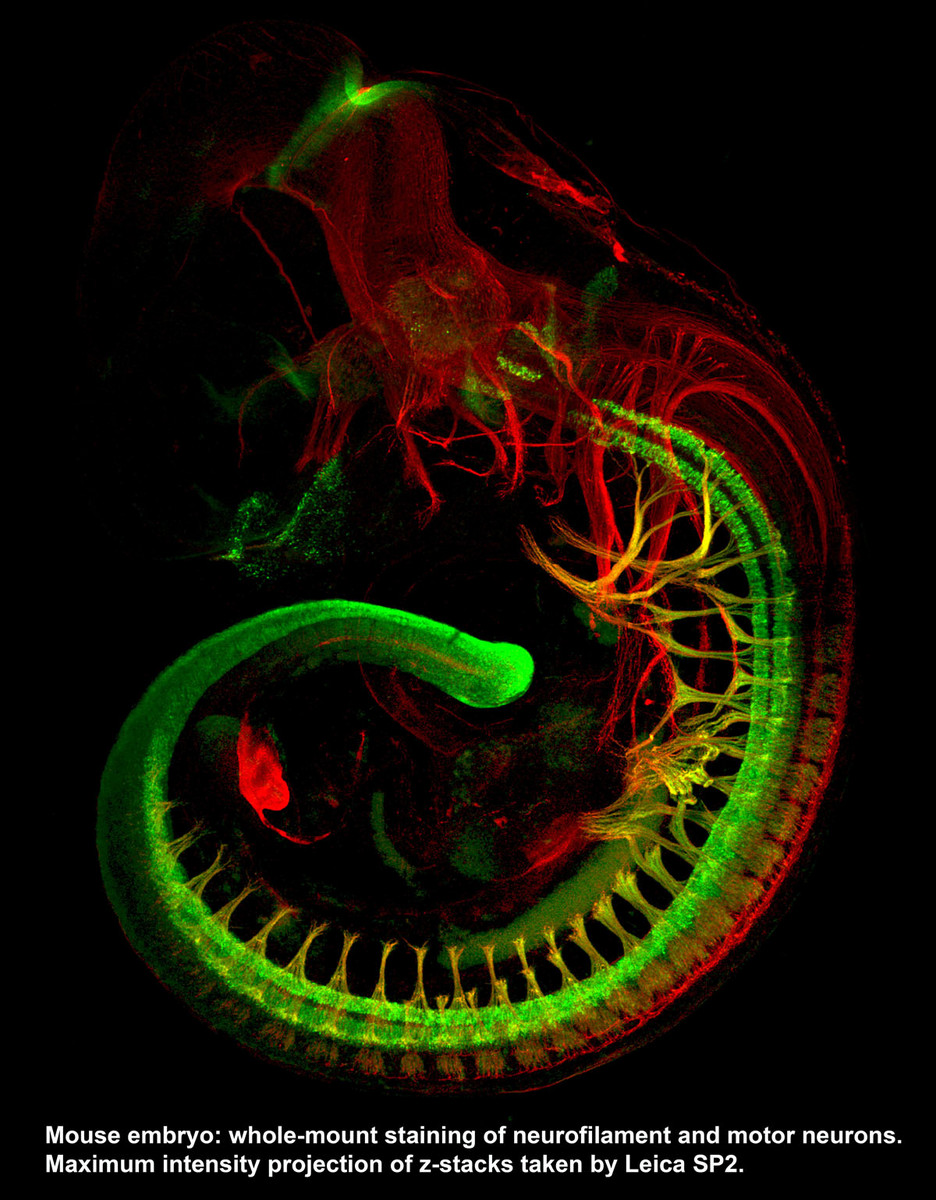 Leica SP8 (Inverted Stand) - P8 is equipped with the white light laser technology that allows the excitation laser to be set to any wavelength between 470 and 670 nm, in addition to the 405 laser for DAPI excitation and the standard argon laser lines of 458, 476, 488, 496 and 514 nm. This means one can optimally excite and cleanly separate any fluorophores. SP8 is also equipped with gating technology which significantly eliminates autofluorescence.
Leica SP8 (Inverted Stand) - P8 is equipped with the white light laser technology that allows the excitation laser to be set to any wavelength between 470 and 670 nm, in addition to the 405 laser for DAPI excitation and the standard argon laser lines of 458, 476, 488, 496 and 514 nm. This means one can optimally excite and cleanly separate any fluorophores. SP8 is also equipped with gating technology which significantly eliminates autofluorescence.- PerkinElmer Spinning Disk (Axiovert 200M inverted stand) - PE is a multi-point spinning-disk scanning confocal microscope that can perform optical sectioning at high-speeds (up to 35 frames per second). Equipped with a highly sensitive EM-CCD camera, Perkin Elmer can detect dim signals from cells that are highly photo-sensitive. It is equipped with an incubation system for long-term live imaging.
- Zeiss LSM 5Live (AxioObserver Inverted Stand) - 5Live is a line-scanning confocal microscope that can perform high-resolution optical sectioning at high speeds. With duo-scan capabilities, this system can bleach and photoactivate while imaging, making it the best option for FRAT, FRAP and FRET experiments. Incubation system is capable of maintaining various CO2 (0% to 20%) and temperature (from -20°C to 80°C) settings.
-
 Widefield Microscopes
Widefield Microscopes
- Zeiss ’Metamorph’ (Axiovert 200M Inverted Microscope Stand - Metamorph Imaging System is dedicated to widefield imaging of live samples. The inverted stand is compatible with most samples including multi-well dishes and chamber slides. The environmental chamber maintains the temperature and CO2 level. The system is equipped with a wide variety of filtersets to optimally capture signals. The microscope is also equipped with sensitive cameras and a Lambda DG-4 illumination system for real-time video imaging and ratio imaging.)
- Zeiss ZEN (Observer Inverted Stand) - ZEN is top of the line live imaging system. It is equipped with dual cMOS camera for simultaneous imaging, EMCCD camera for detection of faint signals, and excitation/emission filter wheels for fast imaging. It is also capable of high-throughput imaging of multi-well plates.
- Two Zeiss Widefield Microscopes With ApoTome (Axioplan2 Imaging Upright Stand) - Two wide-field microscopes are equipped for acquiring brightfield, darkfield, epifluorescent, phase-contrast, DIC and polarized images. Two high-resolution cameras on each microscope (one for brightfield, one for fluorescence) allow you to take the best possible images in either applications. In addition, Zeiss Apotome for structured illumination is available to take optically sectioned images.
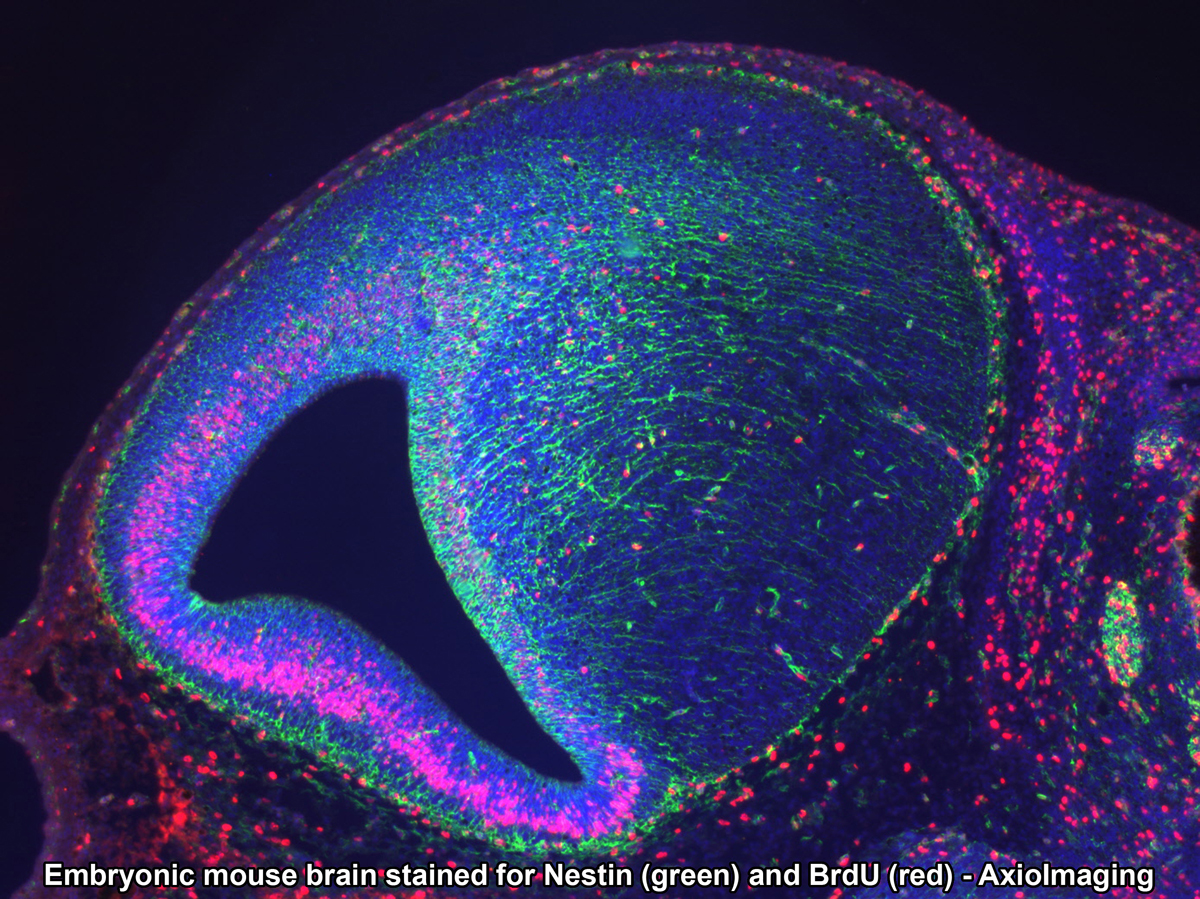 CRI Nuance (Zeiss Axioplan2 Upright Stand) - Nuance is a spectral scanning camera equipped on Zeiss Axioplan 2 upright widefield microscope. This camera has tunable filter that detects signals in spectral range of 420 nm to 720 nm. Multispectral microscopy allows separation of multiple signals with different spectral characteristics. Therefore, fluorophore with very close emission spectra can be distinguished from one another, and autofluorescence can be eliminated from specific signals. This system works not only with fluorescence but also with bright field images with chromatic (color) signals. It allows DAB signal to be separated from Hematoxylin counterstain.
CRI Nuance (Zeiss Axioplan2 Upright Stand) - Nuance is a spectral scanning camera equipped on Zeiss Axioplan 2 upright widefield microscope. This camera has tunable filter that detects signals in spectral range of 420 nm to 720 nm. Multispectral microscopy allows separation of multiple signals with different spectral characteristics. Therefore, fluorophore with very close emission spectra can be distinguished from one another, and autofluorescence can be eliminated from specific signals. This system works not only with fluorescence but also with bright field images with chromatic (color) signals. It allows DAB signal to be separated from Hematoxylin counterstain. 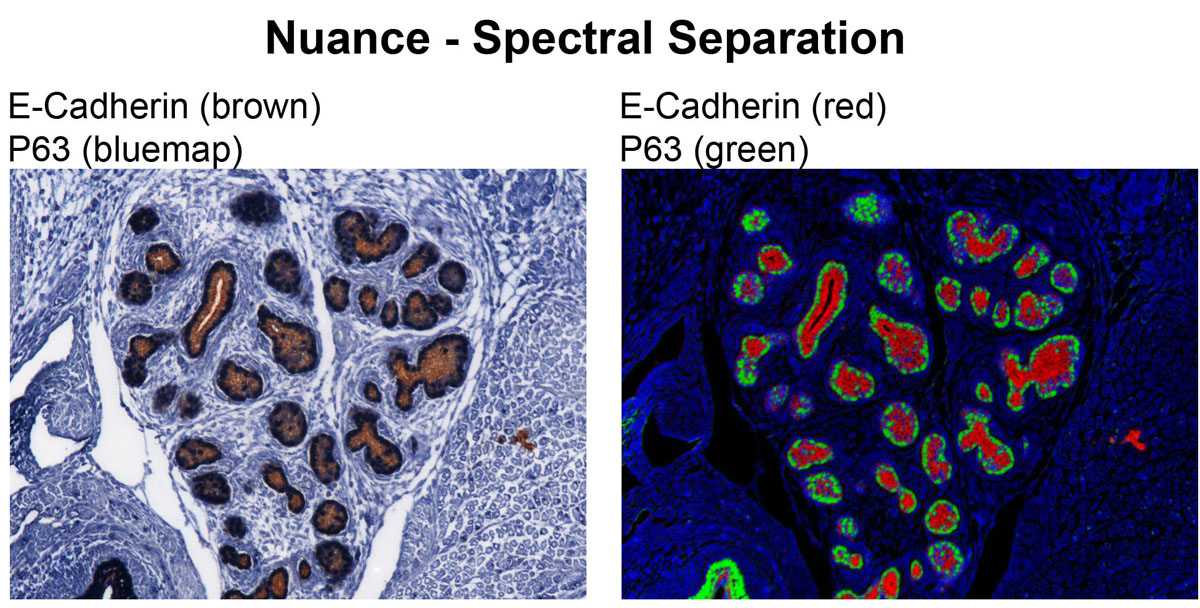
-
Atomic Force Microscopy
- Asylum Research MFP-3D-BIO -
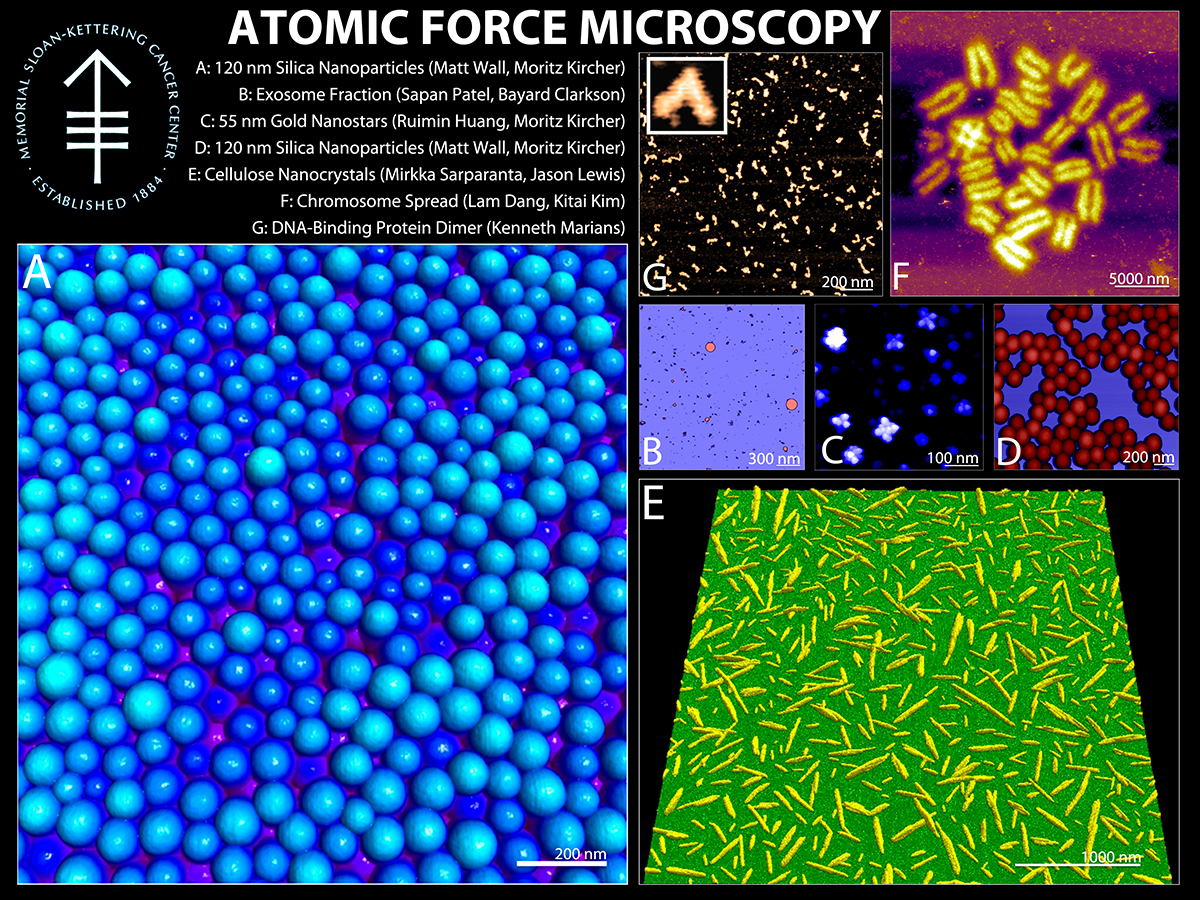 TAFM is an ultra-high resolution imaging technique most often utilized for imaging/measuring nanoparticles, proteins, DNA, viruses, bacteria, organelles, and cells. Structural information is available in 3-dimensions, with angstrom resolution in the Z direction, coupled with nanometer resolution in XY. Force information is available in the pN range. The limitation of AFM is the requirement for the sample to be physically accessible to the AFM probe. More information about AFM is widely available on the internet. The MFP-3D-BIO is optimized to image a wide range of samples, from proteins and DNA to live cells. It can accept tissues, live cells, and other larger samples due to its extended Z-range, heated stage, and open design. It sits on a Zeiss Inverted Optical microscope for simultaneous fluorescent-AFM imaging.
TAFM is an ultra-high resolution imaging technique most often utilized for imaging/measuring nanoparticles, proteins, DNA, viruses, bacteria, organelles, and cells. Structural information is available in 3-dimensions, with angstrom resolution in the Z direction, coupled with nanometer resolution in XY. Force information is available in the pN range. The limitation of AFM is the requirement for the sample to be physically accessible to the AFM probe. More information about AFM is widely available on the internet. The MFP-3D-BIO is optimized to image a wide range of samples, from proteins and DNA to live cells. It can accept tissues, live cells, and other larger samples due to its extended Z-range, heated stage, and open design. It sits on a Zeiss Inverted Optical microscope for simultaneous fluorescent-AFM imaging.
- Asylum Research MFP-3D-BIO -
-
Stereoscopes
 Leica MZFLIII - The integrated ’Combi’ turret allows for fast-switching between low-resolution 3D stereoscopy and high-resolution microscopy. The QImaging Retiga (1360x1036 image array) and integrated LCD filter allow simplified color bright field imaging. This system is also capable of fluorescence imaging.
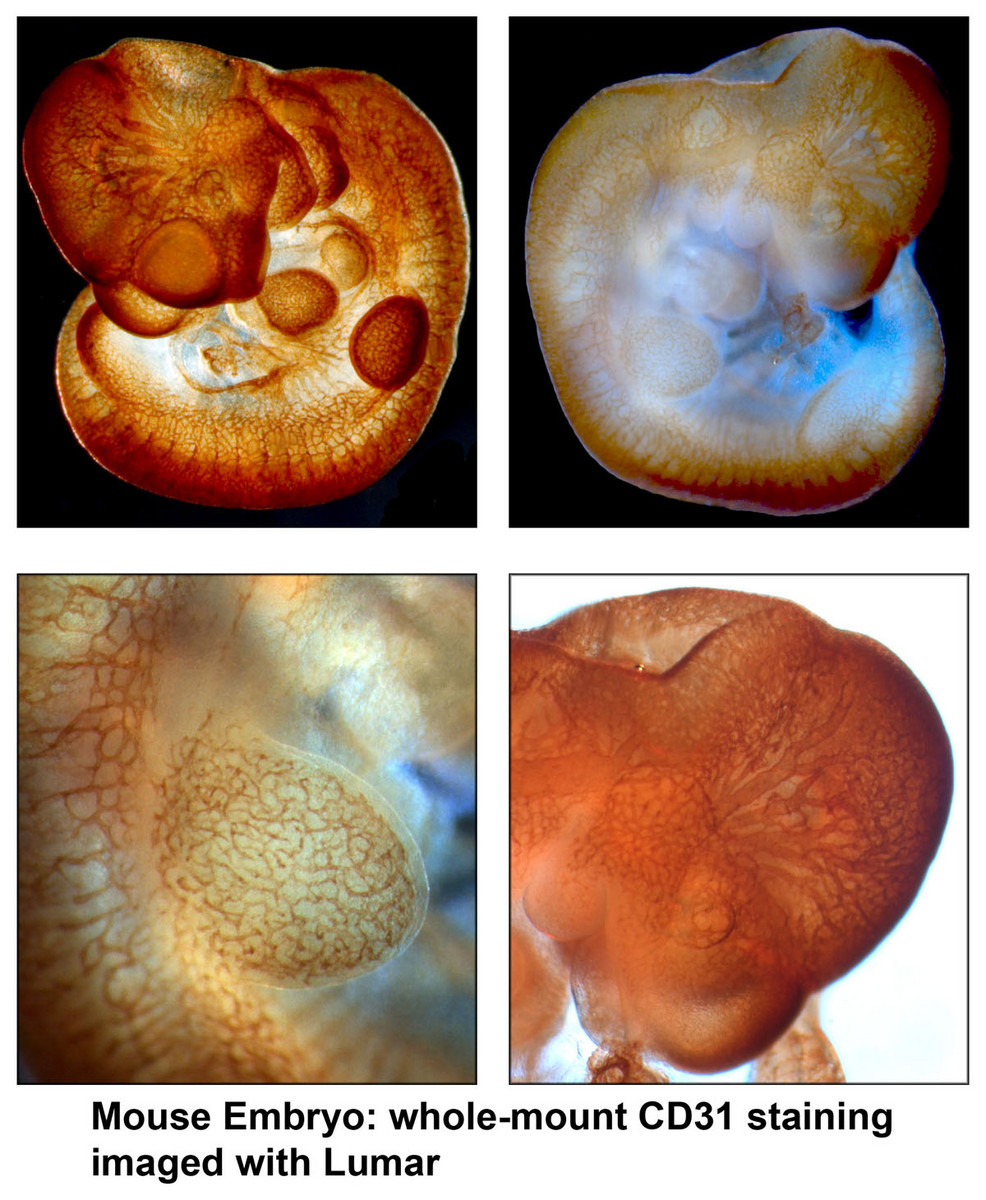
Leica MZFLIII - The integrated ’Combi’ turret allows for fast-switching between low-resolution 3D stereoscopy and high-resolution microscopy. The QImaging Retiga (1360x1036 image array) and integrated LCD filter allow simplified color bright field imaging. This system is also capable of fluorescence imaging.- Zeiss Lumar - Lumar Stereoscope is equipped with high-quality objectives that allow detection of fluorescence and color with great efficiency. The sensitive AxioCam provides high-resolution images for large, whole-mount organ, embryo or organism imaging.
-
Digital Slide Scanner
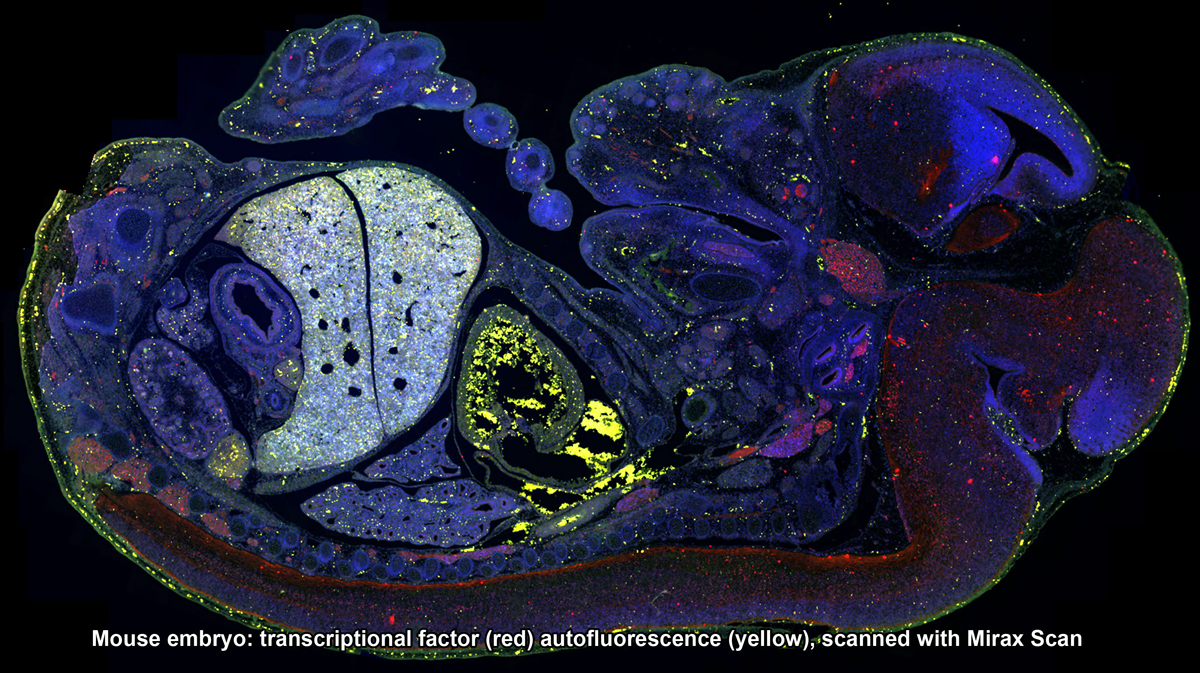
 3DHistech Pannoramic Scanners: SCAN, MIDI and FLASH - The Mirax slide scanners take brightfield or fluorescent slides and completely digitize them. High resolution images are captured with 20x/0.8NA objective, and the final image can be zoomed in to 80x without any loss of image clarity. Mirax Scan can scan up to 150 slides in an automated fashion. Mirax Midi is capable of scanning chamber slides that have been stained and cover-slipped.
3DHistech Pannoramic Scanners: SCAN, MIDI and FLASH - The Mirax slide scanners take brightfield or fluorescent slides and completely digitize them. High resolution images are captured with 20x/0.8NA objective, and the final image can be zoomed in to 80x without any loss of image clarity. Mirax Scan can scan up to 150 slides in an automated fashion. Mirax Midi is capable of scanning chamber slides that have been stained and cover-slipped.
-
Laser Capture Microdissection
- Arcturus Pixell II LCM (Upright Microscope Stand) - Laser capture microdissection is a technique used to isolate very small clusters of cells from a thin tissue section. Viewed under a microscope, the laser will cut out a specific region of interest and remove the wanted cells. This method is most often used to obtain DNA/RNA/protein from small, specific group of cells within a tissue. Because this system requires a lot of preparation, please contact our staff member in advance to discuss the experiment.
-
Image Processing and Analysis
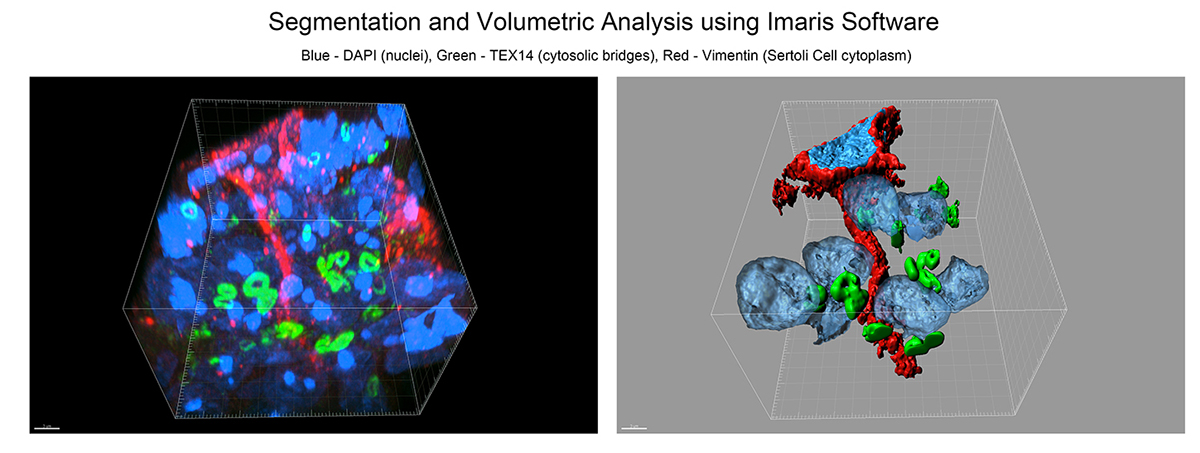 We advise and work with users on all levels of image processing and analysis. For users taking images at our facility, we will advise on most appropriate way to acquire the data set according to the planned analysis. Below is a subset of image processing and analyses that we are able to assist with.
We advise and work with users on all levels of image processing and analysis. For users taking images at our facility, we will advise on most appropriate way to acquire the data set according to the planned analysis. Below is a subset of image processing and analyses that we are able to assist with.
- 3D reconstruction, rendering and volumetric analyses of confocal z-stacks.
- Deconvolution of 2D and 3D data for noise and background reduction.
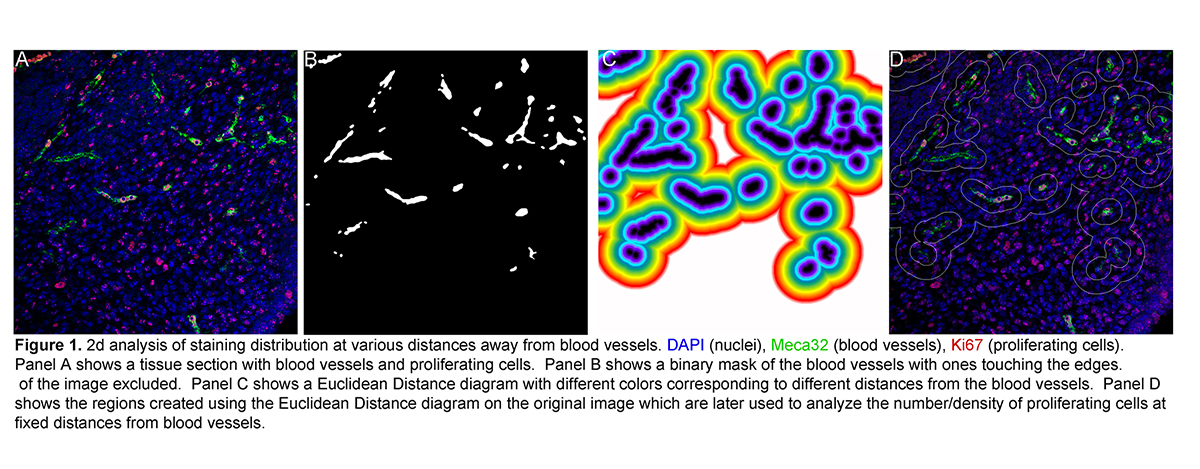
- Particle/cell tracking and movie making of time-lapse data.
- 2D image analyses: co-localization, cell counts, thresholded area measurements, etc.
- Script-writing to automate the analysis procedure.
- Expert training in the use of image analysis software (Metamorph, Volocity, Imaris, AutoDeblur, Voloom, ImageJ/FIJI, MatLab).