Summary of Invention
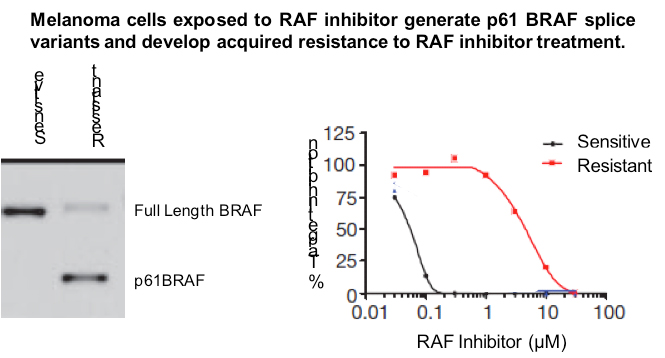
Dr. Neal Rosen and colleagues have identified a novel splice variant of activated BRAF that predicts resistance to continued treatment with RAF inhibitors. Using a cell-line model, the inventors demonstrated that exposure to a RAF inhibitor resulted in the formation of a novel 1.7kb splice variant encoding constitutively active BRAF lacking exons 4-8, which they refer to as p61BRAF. This splice variant was shown to drive resistance to treatment with RAF inhibitors and such inhibition could be overcome by combination treatment with a MEK inhibitor. Retrospective analysis of melanoma patient samples demonstrated that 30% of patients that developed resistance to Zelboraf harbored the p61BRAF splice variant, indicating the clinical relevance of these findings. Further, the inventors have developed reagents and assays useful for identifying novel therapeutics that can overcome RAF-inhibitor resistance.
Advantages
Provides clinically actionable results prior to measureable tumor progression
Stage of Development
Validated in retrospective analysis of patient samples
Publication
Poulikakos, Poulikos (2011) RAF inhibitor resistance is mediated by dimerization of aberrantly spliced BRAF (V600E). Nature. 480:387-390.
Lou, Kai-Jye (2011) Splicing out BRAF’s resistance. SciBx. Volume 4/Number 48: 1-3.
Lead Inventor
Neal Rosen, MD, PhD, Laboratory Head, Molecular Pharmacology Program, Sloan Kettering Institute, Memorial Sloan Kettering
Intellectual Property Status
U.S. Basic Patent issued 9,481,910, in November 2016
Contact Information
Lisa Kennedy, PhD
Senior Business Manager
Email: [email protected]