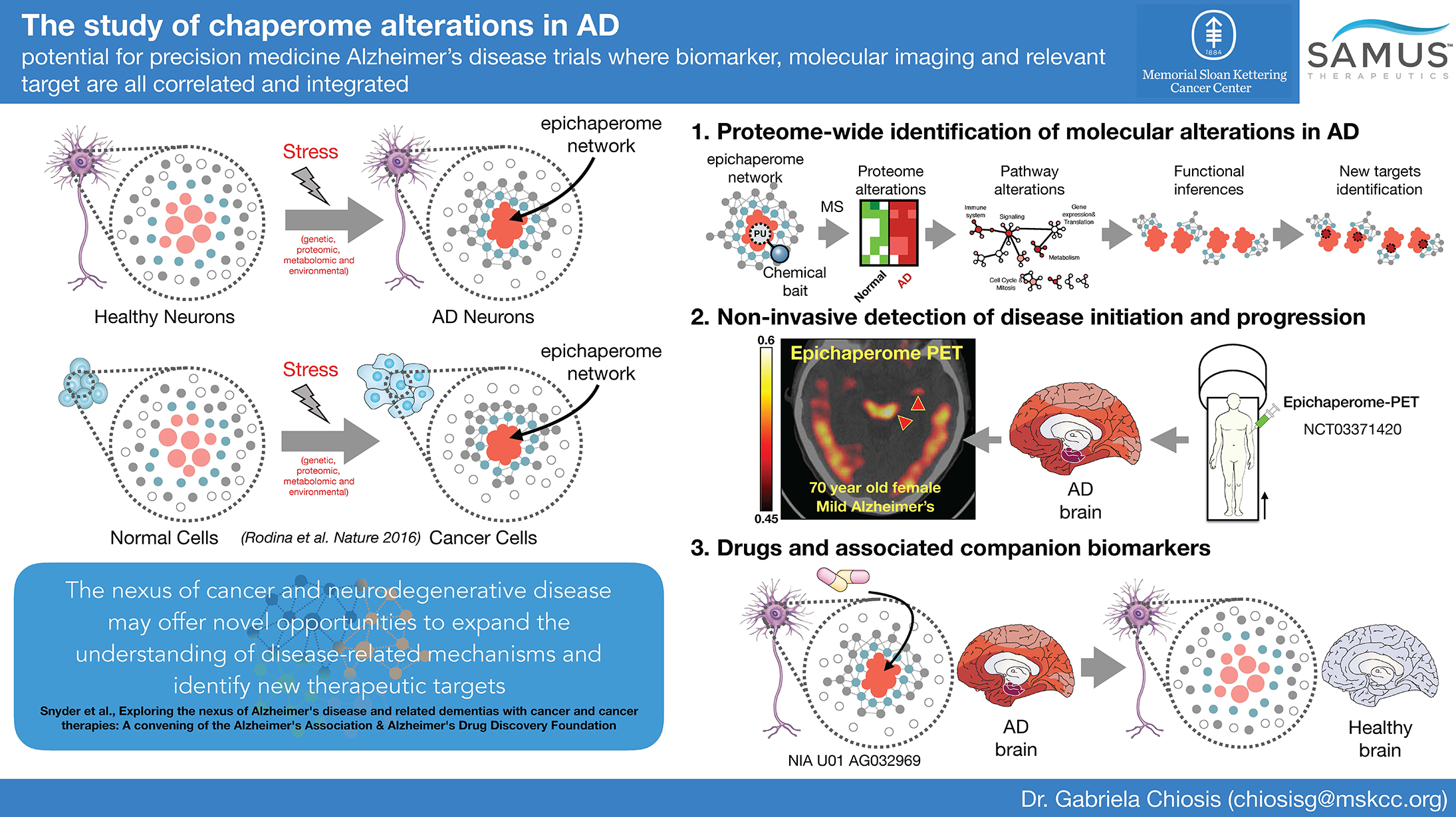
Relevant towards understanding executive function and cognitive processes, we presented evidence, first published in 2016 in Nature and expanded upon in 2018 in Nature Reviews Cancer, that proteome-wide alterations in disease are executed by a restructuring of a fundamental protein group – the chaperome, a collection of chaperones, co-chaperones and other factors – into higher order long-lived assemblies we termed epichaperomes. In 2018 in Nature Communications we provided evidence that epichaperomes act as scaffolding platforms leading to protein-protein interaction alterations in brain cells, such as dopaminergic neurons, exposed to stressors. In 2020, in Nature Communications, we showed how such stressor-induced changes in protein connectivity via epichaperomes are maladaptive and impact intracellular protein functionality altering phenotypes, which disrupt and remodel brain networks ranging from intercellular to brain connectome levels. We showed defects in synaptic plasticity are a pervasive epichaperome-mediated vulnerability of brain cells in response to various genetic, toxic, and environmental stressors. Although the identity of individual proteins impacted by epichaperome formation often differed between stressor conditions, epichaperome-mediated changes in the interaction of these proteins all had functional output imbalances in protein pathways integral for synaptic plasticity.
Linking epichaperome formation to dysfunction in synaptic plasticity induced by a variety of stressors is highly relevant translationally as it provides a final common path for correcting synaptic plasticity defects in a number of neurological conditions, such as neurodegenerative, neurodevelopmental, as well as neuropsychiatric disorders. Importantly, from a therapeutic perspective, dismantling epichaperomes may be envisioned as a viable and potentially transformative therapeutic approach to rewire network dysfunctions and revert brain cells, and in turn brain connectomes, to the pre-stressor, non-pathological states. Accordingly, we discovered and developed a drug candidate PU-AD, published in 2021 in Nature Communications. In preclinical models of tauopathies, PU-AD rebalanced the activity of brain networks involved in memory and learning to their pre-stressor state (Inda et al. Nature Communications 2020). PU-AD is currently in Phase 2 clinical evaluation in Alzheimer’s disease.
Assessing epichaperomes may prove to be a biosensor of the degree of dysfunction in a particular brain cell and brain region and as an early biomarker across the disease spectrum. Specifically, the higher the epichaperome levels, the higher the number of proteins negatively impacted upon, and in turn, the higher the severity of perturbation to the complex network of molecular interactions in affected cells, and in turn in brain connectomes. We thus also developed a positron emission tomography (PET) probe to detect epichaperomes in vivo. In mice and humans, PET imaging confirmed the feasibility of epichaperomes’ detection and quantitation, in addition to demonstrating their localization in relevant affected brain regions but not in relatively spared regions.
In sum, my group has successfully dissected molecular and cellular events in a circuit and network driven systems context to make significant advances in explaining cognitive decline in neurodegenerative disorders. Our discovery is of importance for treatment as it provides a unified avenue for restoration of brain function with precision medicine opportunities for diagnostic and therapeutic development, especially in the context of neurodegenerative disorders such as Alzheimer’s disease where treatment options are currently limited.
Chiosis G. 2018 NIH Alzheimer’s Research Summit: Path to Treatment and Prevention. NIH campus, Bethesda, Maryland, March 1-2, 2018.
#ADSummit18
NIH 2021 Alzheimer’s Research Summit: Path to Precision Medicine for Treatment and Prevention. Virtual meeting. April 19-22, 2021