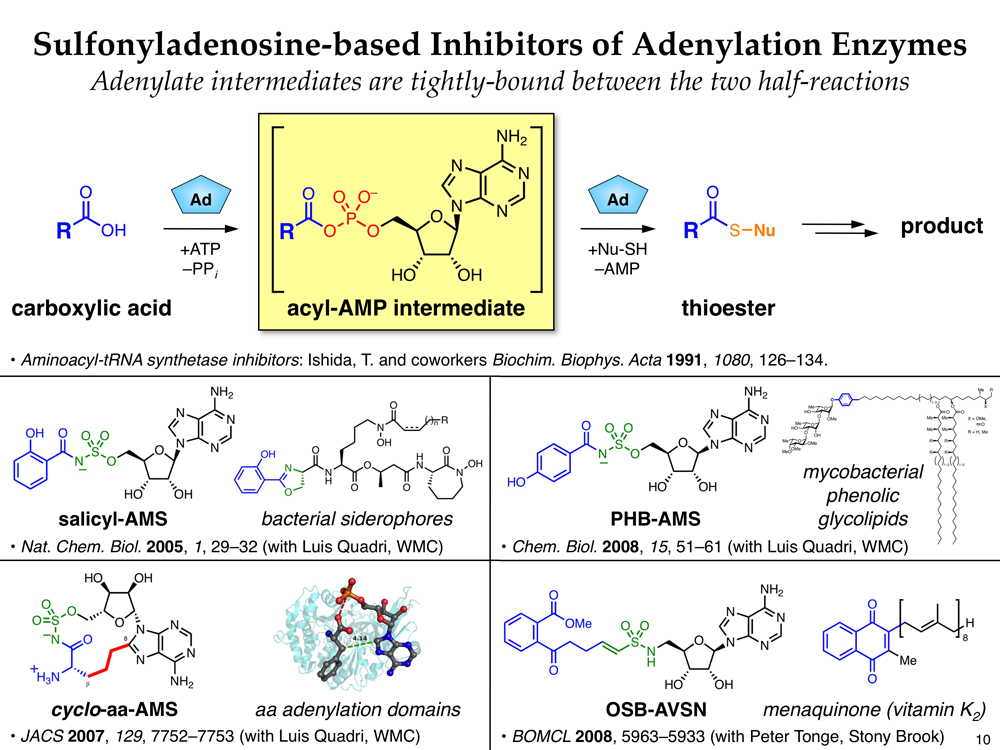
Rational Drug Design Targeting Adenylation Enzymes
Recent evidence suggests that natural products are much more than simply agents of “microbial warfare” and actually play critical roles in bacterial pathogenesis and communication. In particular, non-ribosomal peptide (NRP) natural products have been identified as key players in bacterial iron uptake, biofilm formation, commensalism, and virulence. Thus, small molecule inhibition of NRP biosynthesis provides a powerful means to study the biological roles of these natural products and a potential avenue to develop novel antibiotics. Detailed mechanistic insights into NRP biosynthesis, developed primarily from the perspectives of fundamental interest and engineered biosynthesis, can be leveraged to design such inhibitors.

Salicyl-AMS, a novel adenylation inhibitor developed by rational drug design
In collaboration with Prof. Luis E. N. Quadri at Brooklyn College, we have developed a number of small molecule inhibitors of NRP biosynthesis using natural product-inspired mechanism- and structure-based design. These compounds target enzymes that catalyze key adenylation reactions in the biosyntheses of iron-chelating siderophores and mycobacterial phenolic glycolipids. Recently, in collaboration with Prof. William R. Bishai at Johns Hopkins, we have demonstrated the first in vivo antibacterial efficacy of one of these compounds, salicyl-AMS, in a mouse model of tuberculosis. In collaboration with Prof. Peter J. Tonge at Stony Brook University, we have also developed related inhibitors of menaquinone biosynthesis enzymes as a new class of potential antibiotics.
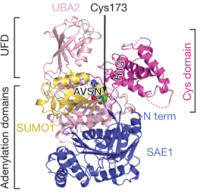
Crystal structure of the SUMO E1 enzyme with SUMO-AVSN, a chemical probe developed by rational drug design
Recently, in collaboration with Prof. Christopher D. Lima at MSKCC, we have used related strategies to develop semisynthetic, mechanism-based protein inhibitors of ubiquitin/ubiquitin-like E1 activation enzymes that regulate diverse biological processes in eukaryotes. This work has revealed striking conformational changes that occur during catalysis and provides new mechanistic insights that may be useful in drug discovery targeting these enzymes.
Publications
Total synthesis of the bacterial diisonitrile chalkophore SF2768.
Xu, Y.; Tan, D. S.* Org. Lett. 2019, 21, 8731–8735.
[ Abstract | PubMed | PMC ]Stereoselective synthesis, docking, and biological evaluation of difluoroindanediol-based MenE inhibitors as antibiotics.
Evans, C. E.; Matarlo, J. S.; Tonge, P. J.*; Tan, D. S.* Org. Lett. 2016, 18, 6384–6387.
[ Abstract | PubMed | PMC ]Design, synthesis, and biological evaluation of α-hydroxyacyl-AMS inhibitors of amino acid adenylation enzymes.
Davis, T. D.†; Mohandas, P. †; Chiriac, M. I.; Bythrow, G. V.; Quadri, L. E. N.; Tan, D. S.* Bioorg. Med. Chem. Lett. 2016, 21, 5340–5345.
[ Abstract | PubMed | PMC ]Designed small-molecule inhibitors of the anthranilyl-CoA synthetase PqsA block quinolone biosynthesis in Pseudomonas aeruginosa.
Ji, C.; Sharma, I.; Pratihar, I.; Hudson, L.; Maura, D.; Guney, T.; Rahme, L. G.; Pesci, E. C.; Coleman, J. P.; Tan, D. S.*; ACS Chem. Biol. 2016, 11, 3061–3067.
[ Abstract | PubMed | PMC ]Mechanism of MenE inhibition by acyl-adenylate analogues and discovery of novel antibacterial agents.
Matarlo, J. S.†; Evans, C. E.†; Sharma, I.; Lavaud, L. J.; Ngo, S. C.; Shek, R.; Rajashankar, K. R.; French, J. B.; Tan, D. S.*; Tonge, P. J.* Biochemistry 2015, 54, 6514–6524.
[ Abstract | PubMed | PMC ]General platform for systematic quantitative evaluation of small-molecule permeability in bacteria.
Davis, T. D.; Gerry, C. J.; Tan, D. S.* ACS Chem. Biol. 2014, 9, 2535–2544.
[ Abstract | PubMed | PMC ]
(Highlighted in ACS Chem. Biol.)Pharmacokinetic and in vivo efficacy studies of the mycobactin biosynthesis inhibitor salicyl-AMS in mice.
Lun, S.; Guo, H.; Adamson, J.; Cisar, J. S.; Davis, T. D.; Sundaramn Chavadi, S.; Warren, J. D.; Quadri, L. E. N.*; Tan, D. S.*; Bishai, W. R.* Antimicrob. Agents Chemother. 2013, 57, 5138–5140.
[ Abstract | PubMed | PMC ]Stable analogues of OSB-AMP: Potent inhibitors of MenE, the o-succinylbenzoate-CoA synthetase from bacterial menaquninone biosynthesis.
Lu, X.; Zhou, R.; Sharma, I.; Li, X.; Kumar, G.; Swaminathan, S.; Tonge, P. J.*; Tan, D. S.* ChemBioChem 2012, 13, 129–136.
[ Abstract | PubMed | PMC ]Active site remodelling accompanies thioester bond formation in the SUMO E1.
Olsen, S. K.; Capili. A. D.; Lu, X.; Tan, D. S.*; Lima, C. D.* Nature 2010, 463, 906–912.
[ Abstract | PubMed | PMC ]
(Highlighted in Nature, Chem. Eng. News, Nat. Rev. Mol. Cell Biol., Nat. Chem. Biol., Structure, ACS Chem. Biol., and Faculty of 1000 Biology )Designed semisynthetic protein inhibitors of Ub/Ubl E1 activating enzymes.
Lu, X.; Olsen, S. K.; Capili, A. D.; Cisar, J. S.; Lima, C. D.*; Tan, D. S.* J. Am. Chem. Soc. 2010, 132, 1748–1749.
[ Abstract | PubMed | PMC ]
(Highlighted in Chem. Eng. News, Nat. Rev. Mol. Cell Biol., ACS Chem. Biol., and Faculty of 1000 Biology )Mechanism-based inhibitors of MenE, an acyl-CoA synthetase involved in bacterial menaquinone biosynthesis.
Lu, X.; Zhang, H.; Tonge, P. J.*; Tan, D. S.* Bioorg. Med. Chem. Lett. 2008, 18, 5963–5966.
[ Abstract | PubMed | PMC ]Small molecule inhibition of microbial natural product biosynthesis – An emerging antibiotic strategy.
Cisar, J. S.; Tan, D. S.* Chem. Soc. Rev. 2008, 37, 1320–1329.
[ Abstract | PubMed | PMC ]Mycobacterial phenolic glycolipid virulence factor biosynthesis: Mechanism and small-molecule inhibition of polyketide chain initiation.
Ferreras, J. A.; Stirrett, K. L.; Lu, X.; Ryu, J.-S.; Soll, C. E.; Tan, D. S.; Quadri, L. E. N.* Chem. Biol. 2008, 15, 51–61.
[ Abstract | PubMed | PMC ]
(Highlighted in Chem. Biol. )Exploiting ligand conformation in selective inhibition of non-ribosomal peptide synthetase amino acid adenylation with designed macrocyclic small molecules.
Cisar, J. S.; Ferreras, J. A.; Soni, R. K.; Quadri, L. E. N.*; Tan, D. S.* J. Am. Chem. Soc. 2007, 129, 7752–7753.
[ Abstract | PubMed | PMC ]
(Highlighted in Faculty of 1000 Biology )Small-molecule inhibition of siderophore biosynthesis in Mycobacterium tuberculosis and Yersinia pestis.
Ferreras, J. A.; Ryu, J.-S.; Di Lello, F.; Tan, D. S.*, Quadri, L. E. N.* Nat. Chem. Biol. 2005, 1, 29–32.
[ Abstract | PubMed ]
(Highlighted in Nature, Nat. Chem. Biol., Chem. Eng. News., and Mercosur Económico )
News Articles
06/01/2010
Collaborative Team Advances the Understanding of an Important Activity Inside Cells
MSKCC Center News
A collaborative team of researchers from Memorial Sloan Kettering has determined the mechanism for a biological process that plays a key role in regulating cellular behavior. The process — and the enzymes that control it — has been studied for 30 years, but until now it was a mystery to researchers in the field how this complex reaction takes place. [Full text]
02/22/2010
Activation of Protein Tags: Enzymology: To prepare biological labels for attachment, E1 enzymes dramatically remodel themselves
Chemical & Engineering News
In a tour de force chemical, structural, and mechanistic study that took five years, researchers have solved a long-standing mystery in a Nobel Prize-winning field of research-they have shown how E1 enzymes activate ubiquitin and related proteins to tag other proteins. [Full text]
02/18/2010
Structural Biology: Transformative Encounters
Nature
Researchers have met the challenge of capturing transient states of the SUMO E1 activating enzyme. Their pictures show radically different crystal structures for two of the steps in this enzyme’s activity. [Full text]
08/18/2008
From Peptides to Polymers: Molecular probes for biological investigation
NYAS eBriefing
Chemical biologists seek to design new chemical tools for use in research and medicine. Their search is predicated on the incredible diversity of chemical structures, both natural and otherwise. This diversity was well represented at the Chemical Biology Discussion Group’s Special Year-End Meeting, held June 2, 2008.
[Overview (free) | Meeting report (membership req’d)]
Justin Cisar’s seminar: Inhibition of Nonribosomal Peptide Synthetase Amino Acid Adenylation Domains
[Video (membership req’d)]
01/01/2008
A Novel Niche Approach to Antibacterial Drug Development
Start-Up Magazine
Researchers are finding new ways to disable bacteria, either by increasing their sensitivity to existing drugs or decreasing their virulence. A group recently reported encouraging, if very early, results about the ability of an inhibitor of virulence factor biosynthesis to control tuberculosis infection. [Full text (subscription req’d)]
12/27/2007
New Drug Targets May Fight Tuberculosis and Other Bacterial Infections in Novel Way
Weill Cornell News
Research into “virulence factors” expands war against infectious disease beyond antibiotics, Weill Cornell researchers say. [Full text]
09/01/2005
New Potential Antibiotic Inhibits Bacterial Growth
MSKCC Center News
Memorial Sloan Kettering Cancer Center and Cornell University researchers have synthesized a molecule impeding the growth of two harmful bacteria: M. tuberculosis, estimated to infect one-third of the world’s population, and Y. pestis, the cause of pneumonic and bubonic plague. [Full text]
06/13/2005
Investigadores elaboran compuesto para combatir bacterias de la tuberculosis y la peste negra
Mercosur Económico
Investigadores de dos prestigiosas instituciones de Nueva York…desarrollaron un compuesto capaz de combatir bacterias -de la tuberculosis y de la peste negra- y permitir la elaboración de nuevas drogas contra una enfermedad que afecta a un tercio de la población mundial -la tuberculosis- y otra en condiciones de ser empleada como arma biológica en bioterrorismo -la peste negra-. [Full text (PDF)]
05/30/2005
A New Way To Fight Bacteria: Inhibitor blocks biosynthesis of key bacterial iron-scavenging agent
Chemical & Engineering News
Full text]
05/26/2005
Chemical Biology: Ironing out bugs
Nature
Researchers from Cornell University and the Memorial Sloan Kettering Cancer Center in New York have devised a molecule that binds to and inhibits enzymes involved in siderophore synthesis. The compound successfully reduces the growth of both Mycobacterium tuberculosis and Yersinia pestis under iron-poor in vitro conditions. [Full text]