When people think of treatments for cancer, they likely think of surgery, radiation therapy, and drugs — both traditional chemotherapy and, more recently, targeted drugs. Increasingly, however, cancer therapies may be vaccines, antibodies, or therapies made from whole cells.
Cell therapies, sometimes called “living therapies,” are an especially promising and rapidly growing area of cancer research. One approach that’s been pioneered by Memorial Sloan Kettering researchers, led by investigator Michel Sadelain, is called CAR T cell immunotherapy. This type of targeted immunotherapy aims to boost the immune system by giving immune cells the information they need to better recognize tumor cells as foreign and attack them.
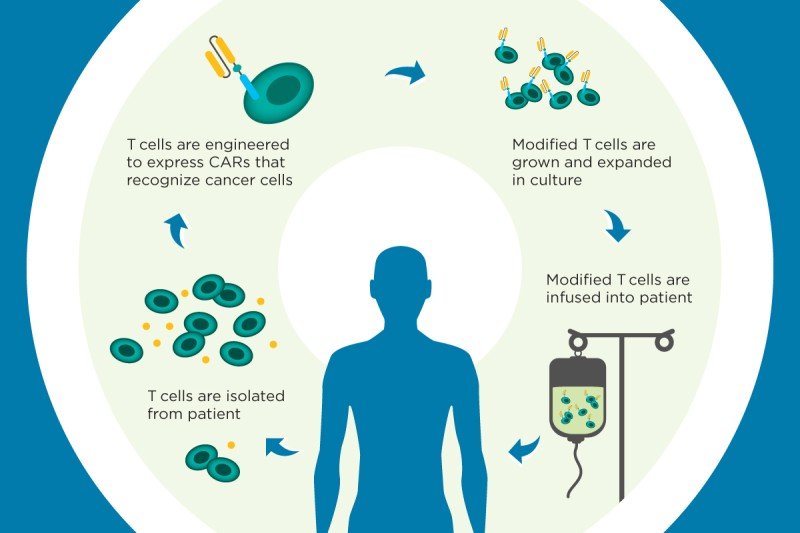
The technique involves filtering white blood cells called T cells from a patient’s blood and introducing a new gene into those cells. A disabled virus called a vector is used to carry the gene inside the T cells and insert it into the cells’ genomes.
The gene programs the T cells to make a chimeric antigen receptor (CAR), which enables them to recognize a specific protein that’s present in cancer cells. The CAR T cells are then grown in the laboratory and infused back into the patient, where they seek out and destroy the cancer.
CAR T cell therapy is currently being evaluated in the clinic at MSK for certain types of leukemia and lymphoma. In this approach, T cells are genetically engineered to recognize a protein called CD19, which is found on the surface of blood cells called B cells. In the largest study reported so far, for adult patients with B cell acute lymphoblastic leukemia — a rapidly progressing form of blood cancer — a report published by MSK researchers last year found that 88 percent of patients responded to the therapy. In late 2014, the US Food and Drug Administration granted MSK Breakthrough Therapy Designation for its CD19 CAR therapy.
CAR therapy is also currently being studied at MSK for the treatment of a type of sarcoma (a cancer of the soft tissues) as well as advanced prostate cancer that has stopped responding to other treatments. Plans are under way to begin trials for other kinds of cancer as well, including tumors of the chest cavity — mesothelioma and certain lung and breast cancers — and ovarian cancer.