Cellular senescence is a stress-response program (Figure 1) that is potentially tumor suppressive. We previously showed that senescence arises through chromatin remodeling that silences proliferative genes while activating genes encoding secreted proteins (1-5). This latter component, termed the senescence-associated secretory phenotype (SASP), has potent effects on the tissue environment that can lead to the immune-mediated targeting of senescent cells (2, 5-7). However, the biological effects of senescent cells are heterogeneous. Whereas we and others have shown that senescence can facilitate wound resolution (8-10), the aberrant persistence of senescent cells in chronically-damaged tissues contributes to cancer progression (11-17) and to a range of non-cancer pathologies (18). Understanding the mechanisms that dictate this diverse biology remains a major goal.
Recent efforts from our laboratory have focused on the roles and regulation of the SASP and its action in immune surveillance of senescent cells (19, 20). Using a well-controlled setting where senescence can be induced in liver tumors by reengaging the p53 tumor suppressor, we showed how remodeling of the surface proteome of senescent cells alters their ability to sense and respond to microenvironmental signals. As one example, senescent cells become hypersensitive to interferon-g and become more effective antigen-presenting cells (21). Together, it appears senescent cells alter, and are affected by, their microenvironment.
Emerging data suggest substantial heterogeneity in the senescence program (19, 22-25) that we plan to systematically characterize using our powerful models, single-cell approaches, and functional perturbations. Beyond identifying better senescence biomarkers, we aim to achieve a mechanistic understanding of factors that dictate senescent cell persistence or clearance (Figure 2).
From a therapeutic standpoint, we are interested in provoking senescence-associated immune surveillance in tumor cells and blunting the deleterious effects of senescent cells in pathologies associated with chronic tissue damage and aging. Hence, we continue to characterize how senescence induction modulates the immune response and are testing new combination strategies based on this understanding. Current studies, in mice, indicate that some drugs that trigger senescence can provoke immune-mediated clearance and/or enhanced response to immune checkpoint blockade (19, 20, 26). A long-term goal is to use our understanding of senescence biology to increase the fraction of cancer patients who benefit from immunotherapy.
There is also substantial interest in ‘senolytic’ drugs that can eliminate senescent cells from damaged tissue. While promising, many current agents are toxic and/or their mechanism of action is unclear. Taking a different approach, we collaborated with Michel Sadelain to produce chimeric antigen receptor (CAR) T cells that target a protein selectively upregulated during senescence. These CAR T cells efficiently eliminate senescent cells from tissues (Figure 3), enhance the activity of senescence-inducing therapy, and ameliorate liver fibrosis (27). More recently we showed that senolytic CAR T cells markedly improved metabolic dysfunction, even when the CAR T cells were delivered prophylactically (28). These studies establish cell-based therapy as a viable strategy to treat senescence-associated pathologies and pave the way to use such therapies for non-cancer diseases. Current studies focus on expanding these efforts and developing second-generation approaches for targeting senescent cells.
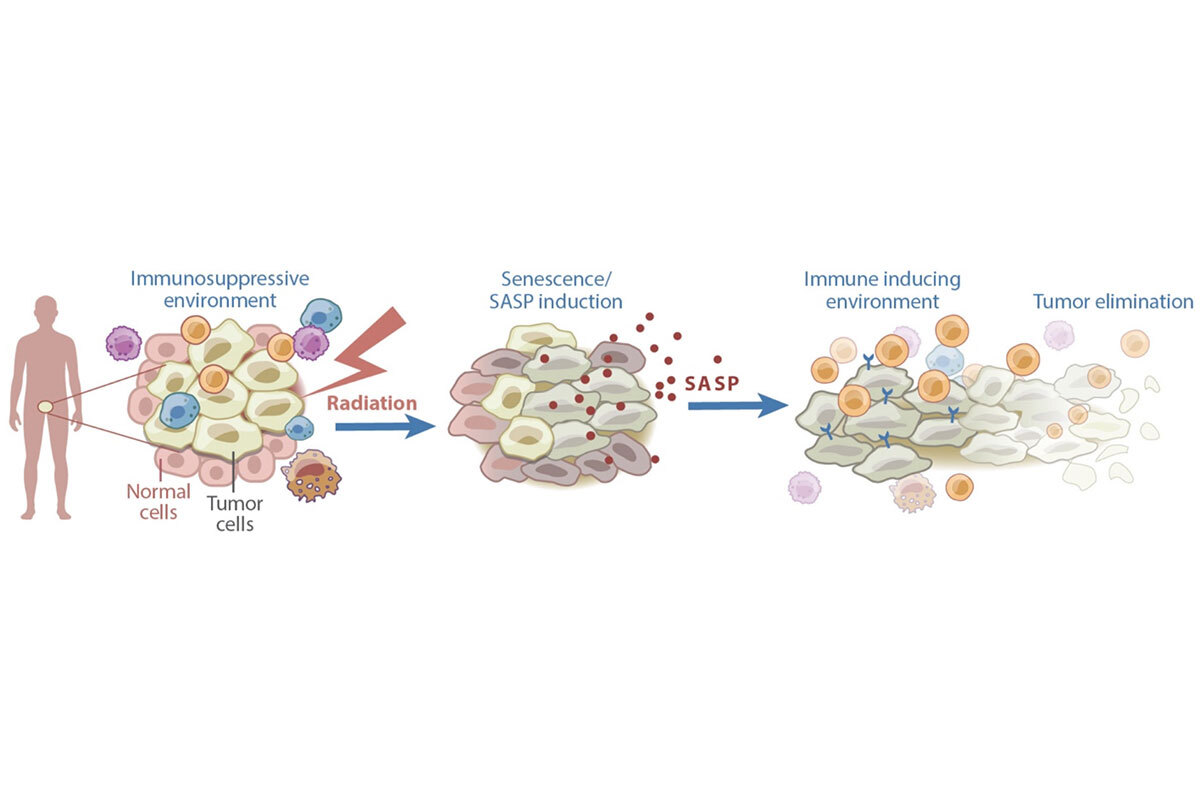
Figure 1. Senescence can contribute to anti-cancer effects of therapy. Certain cancer treatments, including radiation therapy, can induce senescence and the SASP. The SASP subsequently remodels the tumor microenvironment in a manner that induces immune surveillance of the tumor.
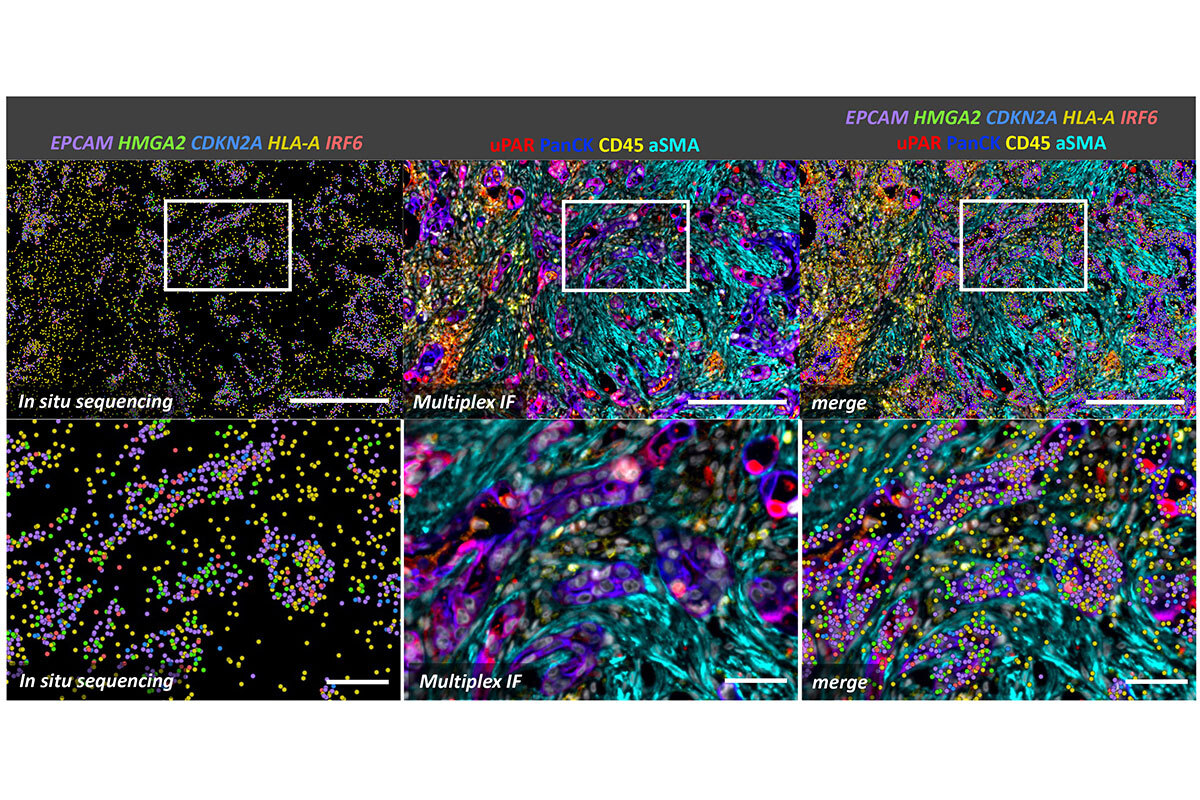
Figure 2. Spatial analysis of senescence at the single-cell level of pancreatic cancer treated with a senescence-inducing chemotherapy. In situ sequencing on the Xenium platform was followed by multiplex immunofluorescence using the CellDive instrument. Senescent cells, characterized by the expression of HMGA2, CDKN2A, and uPAR markers, are enriched among tumor cells (PanCK+ cells). Senescent tumor cells show activation of interferon signaling (IRF6) alongside the expression of MHC I markers (HLA-A), which enhances their susceptibility to immune system recognition. Scale bars: 250 µm in top row and 50 µm in bottom row.
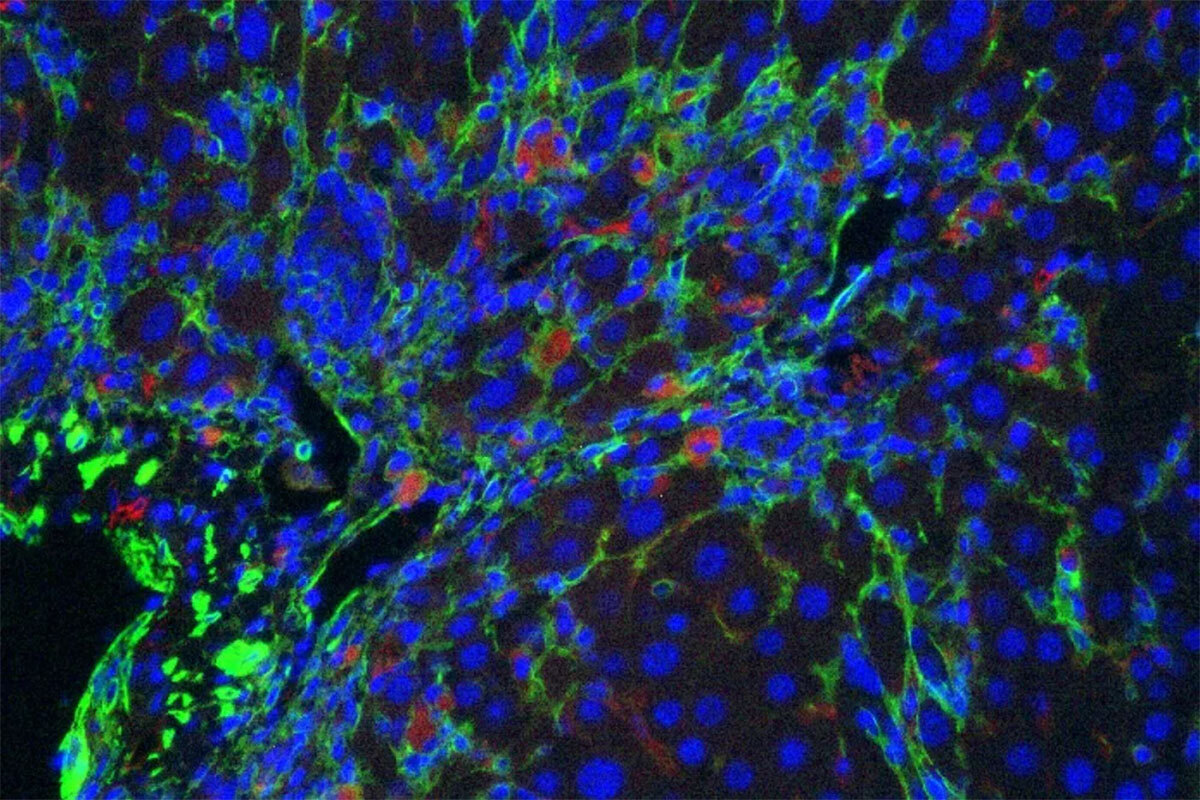
Figure 3. Senolytic CAR T cells. CAR T cells specific for uPAR (red) target senescent cells (green) in a mouse model of liver fibrosis.