Monoclonal antibodies and antibody fragments raised against a variety of tumor-specific and tumor-associated epitopes have been labeled with a variety of radioisotopes for tumor imaging by single-photon emission computed tomography (SPECT) and, more recently, for positron emission tomography (PET) and internal radionuclide therapy. Despite some isolated successes, however, radioimmunodiagnosis and radioimmunotherapy have been limited by circulating antibody’s inaccessibility to the target antigen in solid tumor (on the basal side of the endothelial basement membrane) but ready accessibility to the radiosensitive, dose-limiting hematopoietic marrow (with its characteristic sinusoidal vasculature).
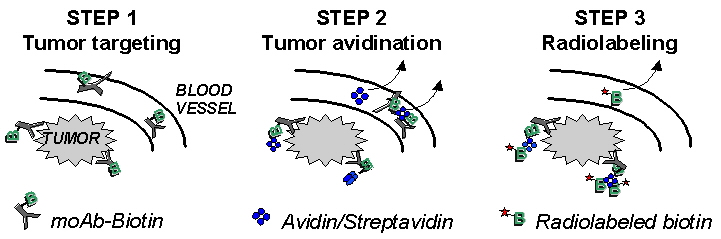
A promising approach to overcoming these limitations is multi-step targeting (MST). Biotin is first attached to the antibody; and the cold biotinylated antibody is injected (Step 1). Sufficient time (several days) elapses to allow the circulating nonradioactive biotynylated antibody to either penetrate the solid tumor and bind to its target antigen or be cleared from the circulation and eliminated. Next, avidin or strepavidin, with a uniquely high affinity for biotin, is injected (Step 2). Additional time (another several days) elapses to allow the circulating nonradioactive avidin/strepavidin to either penetrate the solid tumor and bind to antigen-bound biotinylated antibody or be cleared from the circulation. Finally, radiolabeled biotin, a small ligand, is injected (Step 3) and it is either rapidly cleared (with little localization in and irradiation of normal tissue) or it rapidly penetrates tumor, where it binds to the antigen-bound avidin/strepavidin-biotinylated complex.
Among the projects accomplished in our MST project to date are phantom measurements to investigate the accuracy of 86Y PET imaging as a surrogate for [90Y]-labeled radiopharmaceuticals; development of correction methods to improve the scatter and attenuation correction applied to 86Y imaging; preliminary determination of the comparative biodistribution in tumor-bearing mice of 86Y- and 111In-labeled radiopharmaceuticals (i.e., 2S193 anti-Lewis Y antibody); and preliminary determination of the biodistribution in tumor-bearing mice (with compartmental analysis) of radioiodinated antibody (3F8), radioiodinated strepavidin (the “second” step in 3-step antibody targeting), and 111In-biotin (the “third” step in 3-step antibody targeting). The first 2 areas represent important physical results, which provide and validate the technical basis of the biological studies proposed. We have shown that, with appropriate calibrations and corrections, high-quality quantitative PET can be performed with a radionuclide, such as 86Y, which has less than 100 percent positron emission and many associated single gamma and x-ray emissions (sometimes in cascade with the positron and/or each other).
We have also successfully completed a series of biological (i.e., animal) studies comparing the biodistribution of [111In]- with [86Y]-LewisY antibody. This study confirmed that 111In is a good surrogate isotope for the isotopes of yttrium, especially at early time points (<24 hours) post injection. However, the lower stability of the CHX-A-DTPA chelate for 86Y relative to 111In resulted in a progressively higher ratio of the 86Y radiometal to the parent compound. The marginally slower clearance kinetics of the yttrium radiometal resulted in a progressively higher %ID/g at 48 hours relative to 111In. This indicates that dosimetry estimates based on 111In images would underestimate the doses received by 90Y. For this reason, 86Y would be a more accurate surrogate for 90Y.
Furthermore, the ability to perform quantitative PET imaging with 86Y radiopharmaceuticals offers a significant advantage over 111In. Although the 13.6-hour half-life of 86Y is much shorter than that of 111In (2.7 days), the far higher sensitivity of PET cameras should permit imaging out to 4 half-lives (~2 days). We have demonstrated the feasibility of radiolabeling the LewisY antibody with 86Y, obtaining equivalent tumor uptake (30%ID/g) to 111In, and have been able to obtain high-quality PET images at 48 hours after injection with excellent tumor localization.
The monoclonal antibody 3F8, directed against ganglioside GD2, has been utilized for both imaging and therapy of neuroblastoma. A study was performed
- to determine if 3F8 also can be successfully applied for multi-step radioimmunotargeting, despite internalization and catabolism of 3F8, and
- to evaluate multi-step tumor targeting using biotinylated 3F8 followed by streptavidin and 111In-biotin.
Beginning with in vitro 125I-3F8 cell binding, dissociation, and catabolism data, separate biodistribution data for 125I-3F8, 125I-streptavidin, and 111In-biotin in nude mice, bearing LAN-1 tumors, were fitted to their respective nonlinear compartmental models. Based on analysis of our in vitro 125I-3F8 cell binding data, the overall rate of internalization, catabolism, and iodotyrosine release of antigen-bound monoclonal antibody was 0.018/hour; but the rapid in vivo clearance of iodotyrosine, as well as of free antibody, indicate that virtually all tumor radioactivity represents tumor cell-bound antibody accessible to multi-step targeting.
We anticipate that nonlinear compartmental analysis can be helpful in efficiently designing and optimizing clinical trials of multi-step radioimmunotherapy. (This work was performed in collaboration with Dr. Nai-Kong Cheung of the Department of Pediatrics.)
We now plan to apply MST in an experimental nude mice model, using a highly specific CC49-derived with a novel clearing agent developed by the NeoRx Corp to reduce normal tissue uptake and a stable DOTA-Biotin conjugate. The positron-emitting isotope 86Y will be used for quantitative imaging with PET. The biodistribution and image quality will be compared with a 111In-labeled monoclonal antibody using SPECT. The PET radionuclide 86Y is chemically identical to the therapeutic radionuclide, and thus has an important advantage relative to 111In (commonly used as a surrogate for 90Y) for imaging the biodistribution of 90Y.

