The facility intersects many clinical and research areas including cancer biology, medicine, chemistry, physics, radiochemistry, pharmacology, and engineering. As such, it is one of the largest manufacturing units at Memorial Sloan Kettering in terms of the number of clinical and research products it produces.
Our Services
Radiopharmaceutical Development and Clinical Translation
RMIP manages all chemistry, manufacturing, and controls (CMC) aspects of novel investigational agents during Investigational New Drug (IND) application submissions. The scope of these services includes, radiolabeling method development and optimization, formulation development, analytical method development, standard operating procedures (SOP’s) generation and implementation, production process validation, CMC section generation, and generating CMC-related correspondence with the FDA. In addition, RMIP guides clinical trial principal investigators on all aspects related to the use of investigational agents described in the clinical protocols, including dose determination, patient specific unit dose dispensing, and administration to study subjects.

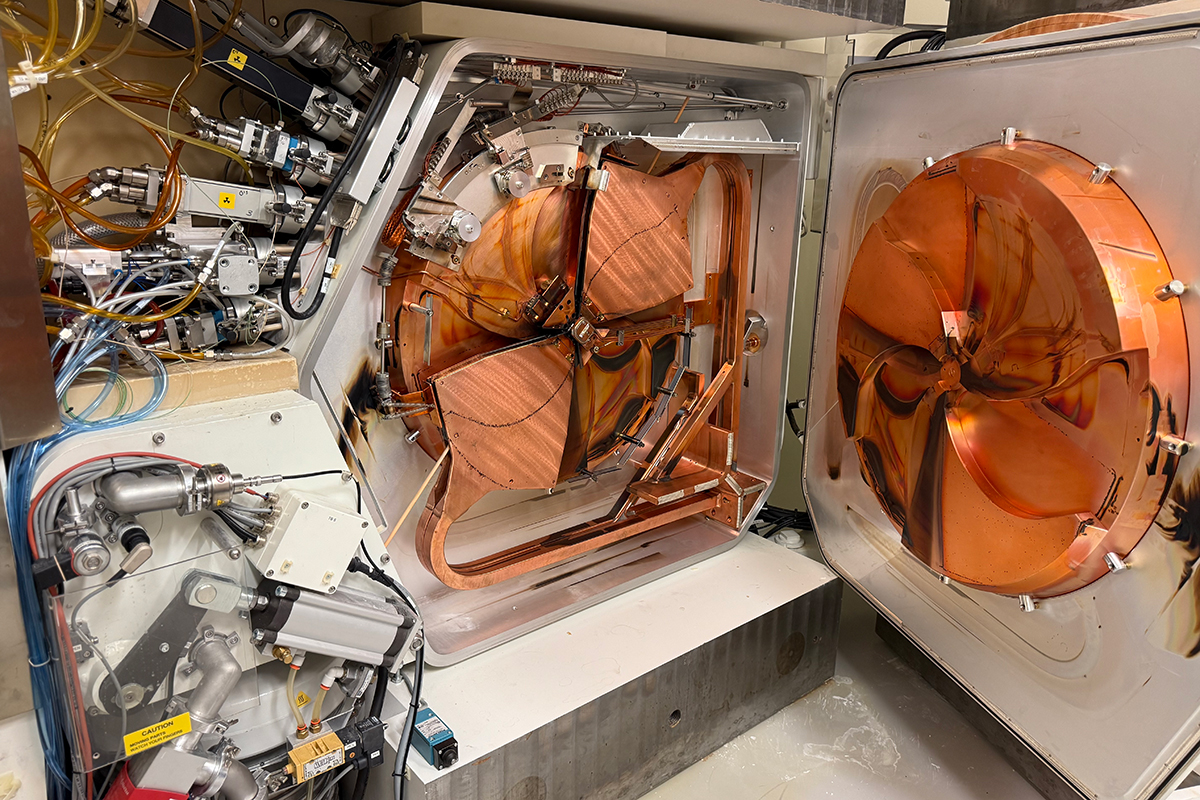
Medical Radionuclide Production in the Cyclotron Facility
RMIP operates a GEMS PETtrace-880 16.5 MeV cyclotron, equipped with both liquid targets and solid targetry systems. The cyclotron was installed in Memorial Hospital in 2013 to enhance MSK’s basic science and clinical translation research programs that rely on PET nuclides. The fully automated machine is routinely used to produce 18F using the liquid targets, and 89Zr, 64Cu, 68Ga, and 61Cu using the solid targetry systems.
Radiopharmaceutical Production – Small Molecules and Peptides
The Small Molecule and Peptide Radiochemistry Section incorporates cyclotron-produced positron-emitting radionuclides into precursor molecules to make radiopharmaceuticals used as diagnostic agents in combination with PET imaging. These radiopharmaceuticals are designed to detect primary and metastatic tumors and provide better delineation of tumor boundaries. The powerful combination of radiolabeled tracers and PET imaging can provide information on functional changes well ahead of the detection of structural changes by imaging tools such as x-ray or CT. Radiolabeled pharmaceuticals, which decay with time, are synthesized individually on the premises for both research and clinical studies. Additionally, RMIP specializes in production of radiotherapeutic investigational agents used in first-in-human clinical trials at MSK. The therapeutic radionuclides, including 177Lu, 161Tb , 225Ac, 228Th, 67Cu, and 131I, are sourced from a global network of nuclear reactors and high energy accelerators.


Radiopharmaceutical Production – Antibodies and Antibody Fragments
The Antibody Labeling Section is responsible for the radiolabeling of monoclonal antibodies and antibody fragments for cancer diagnostic (radioimmunoimaging) and therapeutic (radioimmunotherapy) purposes. RMIP possesses the necessary expertise and equipment that allows for both chemical conjugation of macromolecules with bifunctional chelators, enabling subsequent radiolabeling with diagnostic and therapeutic radionuclides of choice.


Hyperpolarized Magnetic Resonance Imaging Section
RMIP conducts clinical preparation of hyperpolarized MRI agents on MSK’s 5T SpinLab Hyperpolarizer. The process involves reagent loading onto fluid paths, hyperpolarization, dose planning and dispensing, quality control testing, and batch release. The hyperpolarized investigational agents produced at RMIP allow MRI researchers and radiologists to image tumor metabolism in real-time.
Professional Training Program
The RMIP Core Facility offers a professional training program to summer interns, as well as to pharmacist candidates from accredited schools of pharmacy. The scope covers the following:
- Preparation of chemistry, manufacturing, and control (CMC) documentation and submitting of investigational new drug (IND) applications for new radiopharmaceuticals
- Manufacturing radiopharmaceuticals in compliance with cGMP regulatory requirements
- Working in controlled GMP environments (cleanrooms) and utilizing aseptic techniques
- Quality assurance for radiopharmaceuticals prepared for both clinical use and clinical research use.
- Quality control (release testing) for radiopharmaceuticals prepared for human use
- Developing and validating new analytical methods
- Developing, implementing, and managing standard operating procedures (SOPs)
- Dispensing radiopharmaceuticals
- Producing novel radionuclides
- Conjugating and radiolabeling monoclonal antibodies
This program can be tailored toward individual needs. For more information please contact RMIP Core Director, Serge K. Lyashchenko.
Regulatory Compliance
Regulatory Compliance
RMIP Core is New York State Registered Nuclear Pharmacy. Manufacture of investigational agents is conducted as per FDA-acknowledged Investigational New Drug Applications and in compliance with the relevant cGMP and radiation safety requirements.