We are interested in metastasis, the cause of 90% of cancer deaths. The lab investigates metastatic stem cells and their stromal niches. The role of immune regulatory and regenerative signals, particularly TGF-β, is of interest. General as well as organ-specific mediators of metastasis are under investigation. We are defining the phenotypic plasticity and evolution of metastatic cell populations from dormancy to outbreak, identifying drug targets, and enabling clinical trials to treat metastasis.
Stem Cell Signaling, Growth Control, and Cancer Metastasis

TGF-β signaling in development and disease
Transforming growth factor β (TGF-β) signaling plays crucial roles in metazoan development, tissue homeostasis, wound healing, and immune surveillance. Its malfunctions cause developmental defects, immune disorders, fibrosis, and cancer. We defined a pathway in which TGF-β binds to receptor serine/threonine kinases that phosphorylate SMAD transcription factors to regulate gene expression in a context-dependent manner, giving rise to diverse effects in virtually all cell types. The lab is investigating how cells interpret TGF-β signals depending on the presence of context-dependent SMAD cofactors and chromatin configurations at specific target loci. We are focusing on the regulation of growth and phenotype in embryonic and metastatic stem cells, mesenchymal cells and immune cells in the tumor microenvironment. The work incorporates signal-dependent interaction of transcription factors with target genomic loci, chromatin determinants on these interactions, and gene expression programs underlying the effects of TGF-β in these cells. Collaborators include structural biologists and developmental biologists. Our goal is to find treatments for TGF-β driven tumorigenesis and fibrosis.
Phenotypic plasticity regulation
Epithelial-to-mesenchymal transitions (EMTs) are phenotypic plasticity processes that confer migratory and invasive properties to epithelial cells during development, wound-healing, fibrosis and cancer. TGF-β is a potent inducer of EMTs. Aberrant TGF-β signaling and EMT are implicated in renal fibrosis, alcoholic liver disease, non-alcoholic steatohepatitis, pulmonary fibrosis, and cancer. We are elucidating how TGF-β signals coordinately trigger EMTs in concert with pathophysiological processes.
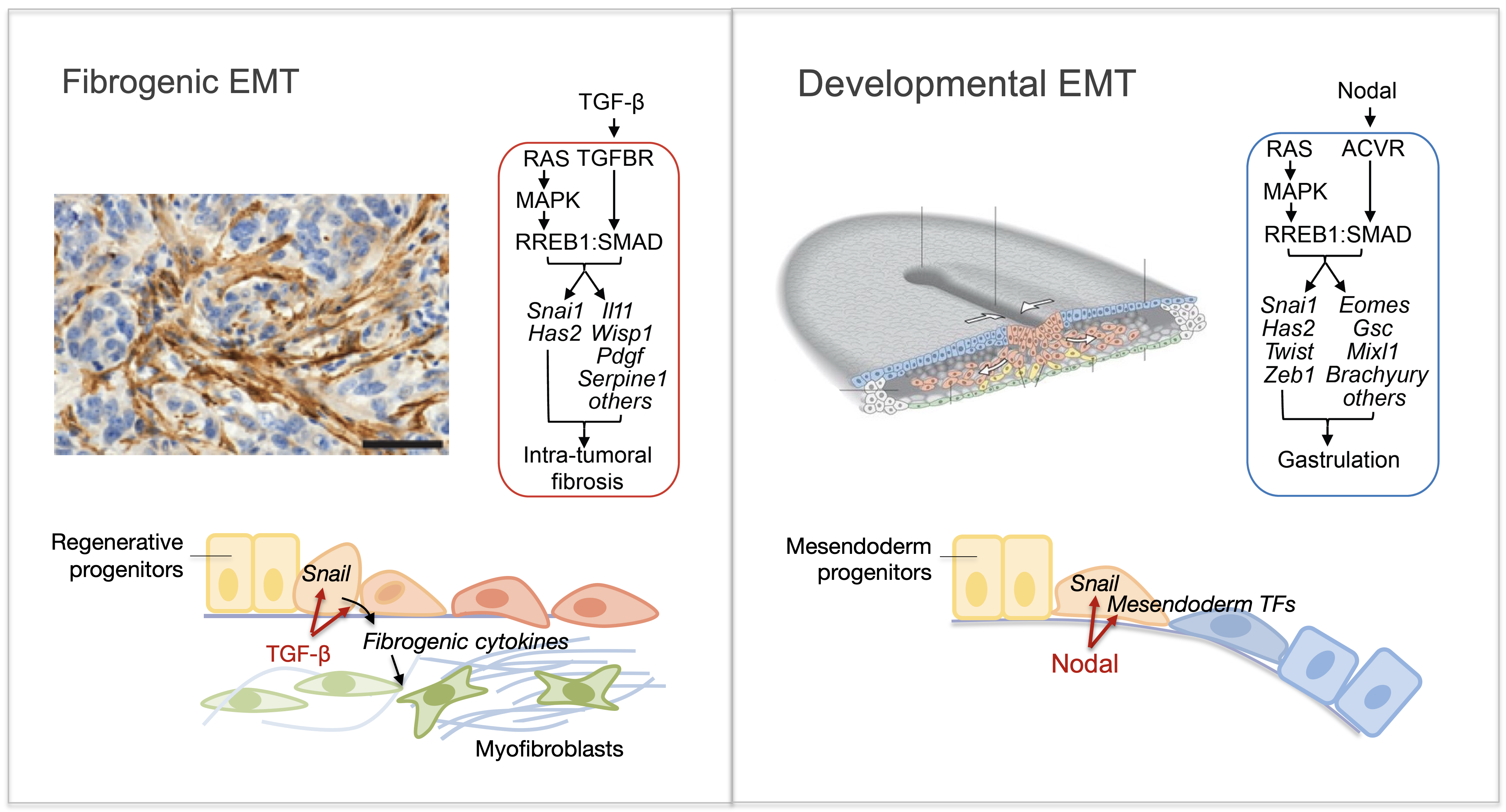
The lab recently identified the RAS transcriptional effector RREB1 as a key partner of TGF-β-activated SMAD transcription factors in the induction of EMT. We are undertaking a thorough analysis of this relatively obscure transcription factor through molecular, structural, and genetic approaches. Context-dependent chromatin accessibility determines the developmental or fibrogenic nature of the resulting EMTs in embryonic cells and carcinoma cells, respectively. We are building on these insights to gain a better understanding of epithelial plasticity regulation by TGF-β in development, fibrosis and metastasis.
Metastasis initiating cells
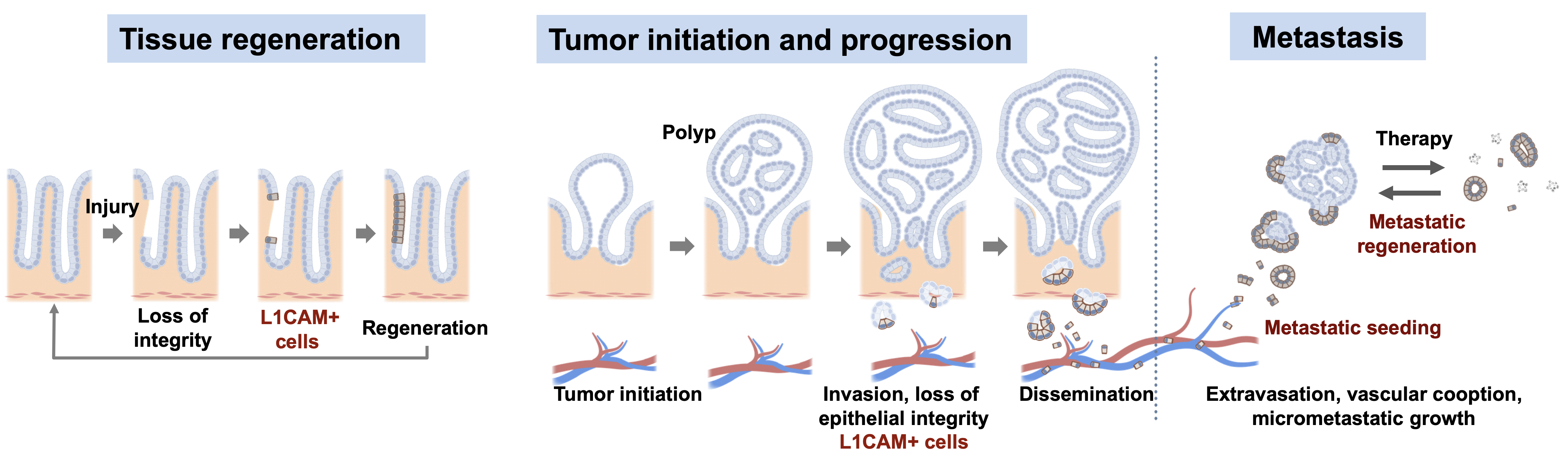
Tissue homeostasis is maintained by stem cells, whereas damaged tissues are repaired by facultative progenitors that are activated upon injury. Developmental processes underlying normal tissue regeneration also operate in metastasis. Metastasis remains the main cause of death from cancer. The persistence and lethal relapse of disseminated cancer is driven by stem-like metastasis initiating cells. We are interested in understanding how the metastasis initiating phenotype emerges during tumor progression. The cell adhesion molecule L1CAM is a marker of the metastasis initiating phenotype in breast, lung, colorectal and renal carcinomas. L1CAM expression is silent in normal epithelial tissues but is active and required during wound healing. Metastasis initiating cells require L1CAM for colonization of multiple organs (brain, lung, lives and bone). Disseminated cancer cells use L1CAM to spread on blood capillaries and activate mechano-transduction transcription factors for tumor outgrowth. Using patient tumor tissues, organoids, mouse models, lineage tracing, advanced imaging, and single cell analytics, the lab is investigating the nature of metastasis initiating cells and their stromal niches and evaluating L1CAM and related molecules as therapeutic targets.
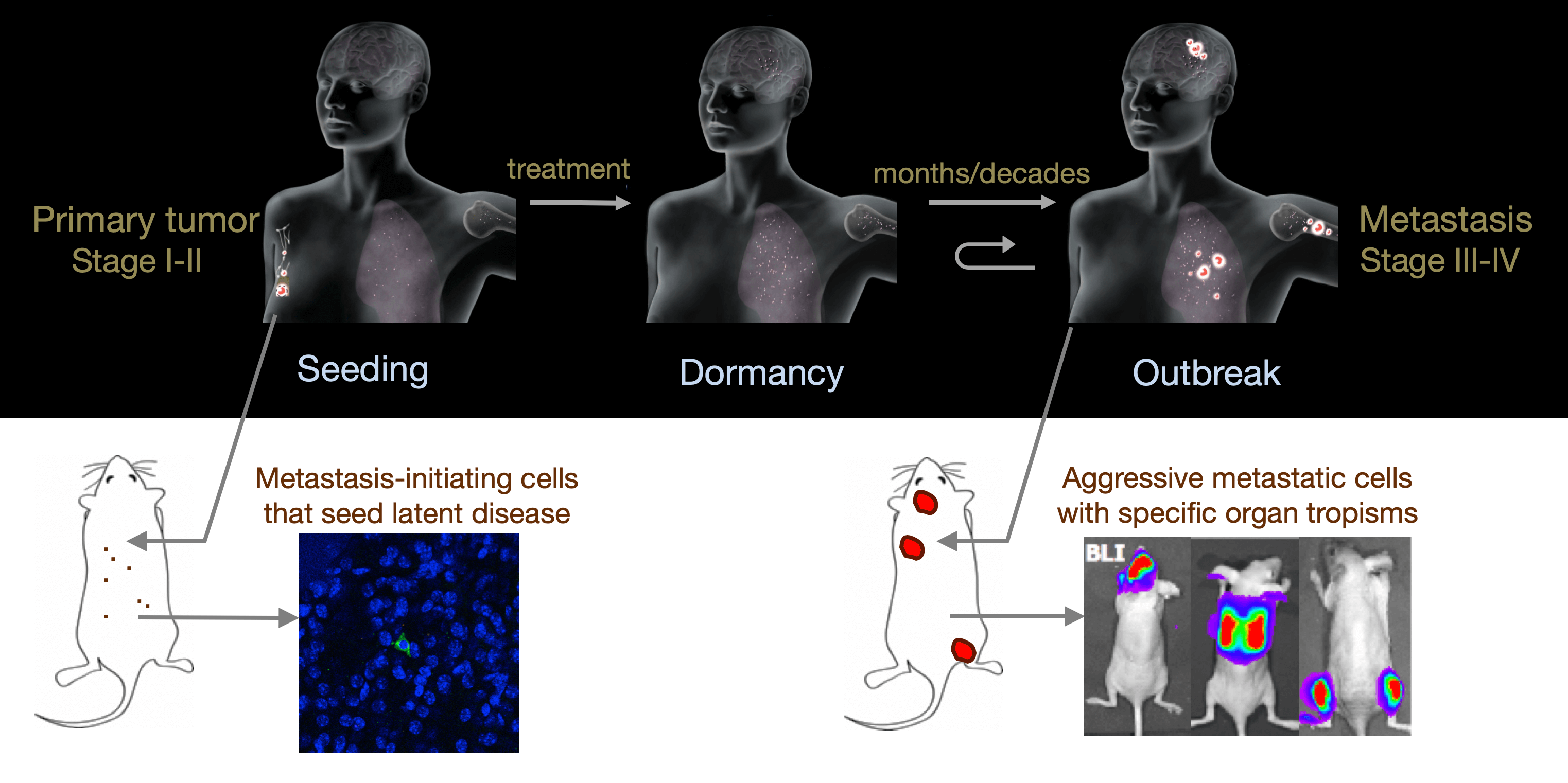
From metastatic dormancy to outbreak
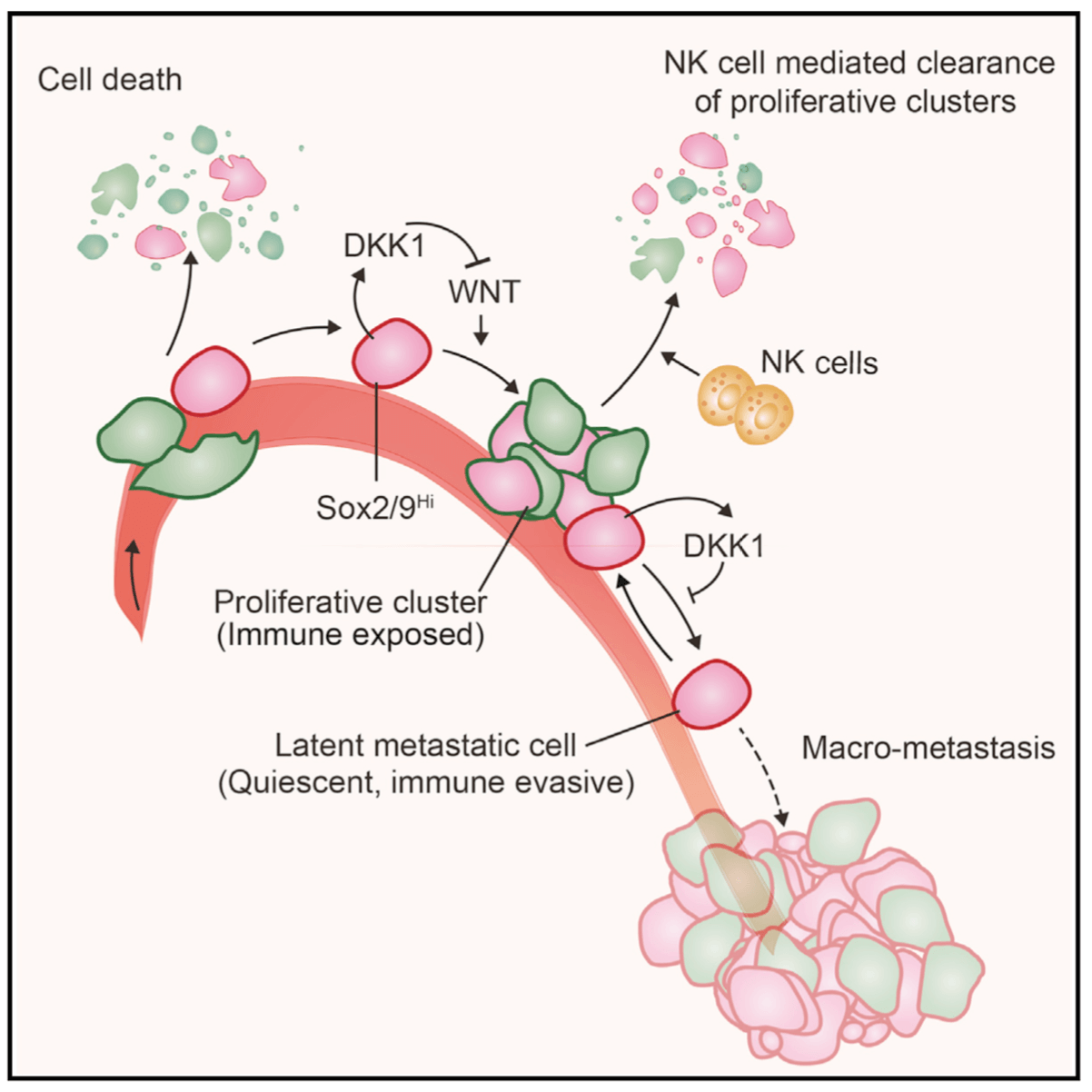
We are defining the evolution of metastasis stem cells that enter a dormant phase after infiltrating target organs, remain viable under immune surveillance, and eventually reinitiate tumor growth. Using unique mouse models that the lab developed, we are defining how lung adenocarcinoma metastasis become enriched for key endoderm and lung-specifying transcription factors and recapitulate transcriptional programs in primitive stem cells and regenerative pulmonary epithelial progenitor states. We are using single-cell analytics and graph-based phenotypic analysis in human tumor tissue samples and mouse models to explore tumor cell heterogeneity from the perspective of epithelial regeneration in metastasis, and to assess parallels between tumor cell plasticity and developmental hierarchies. By defining the intimate relationship between lineage-determining transcriptional programs and immune vulnerabilities during metastatic evolution we hope to identify new therapeutic strategies to suppress metastasis.
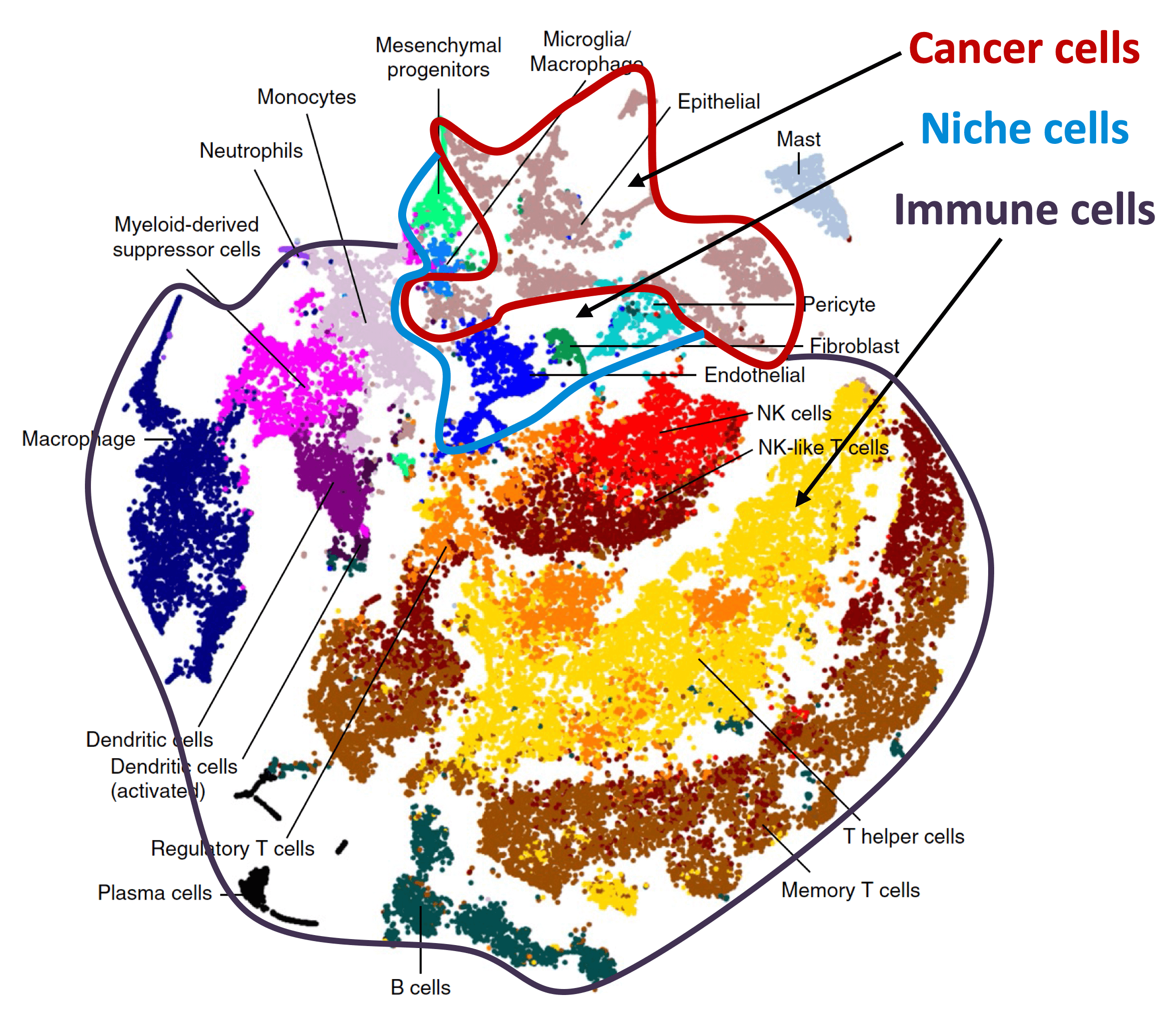
Organ-specific metastasis
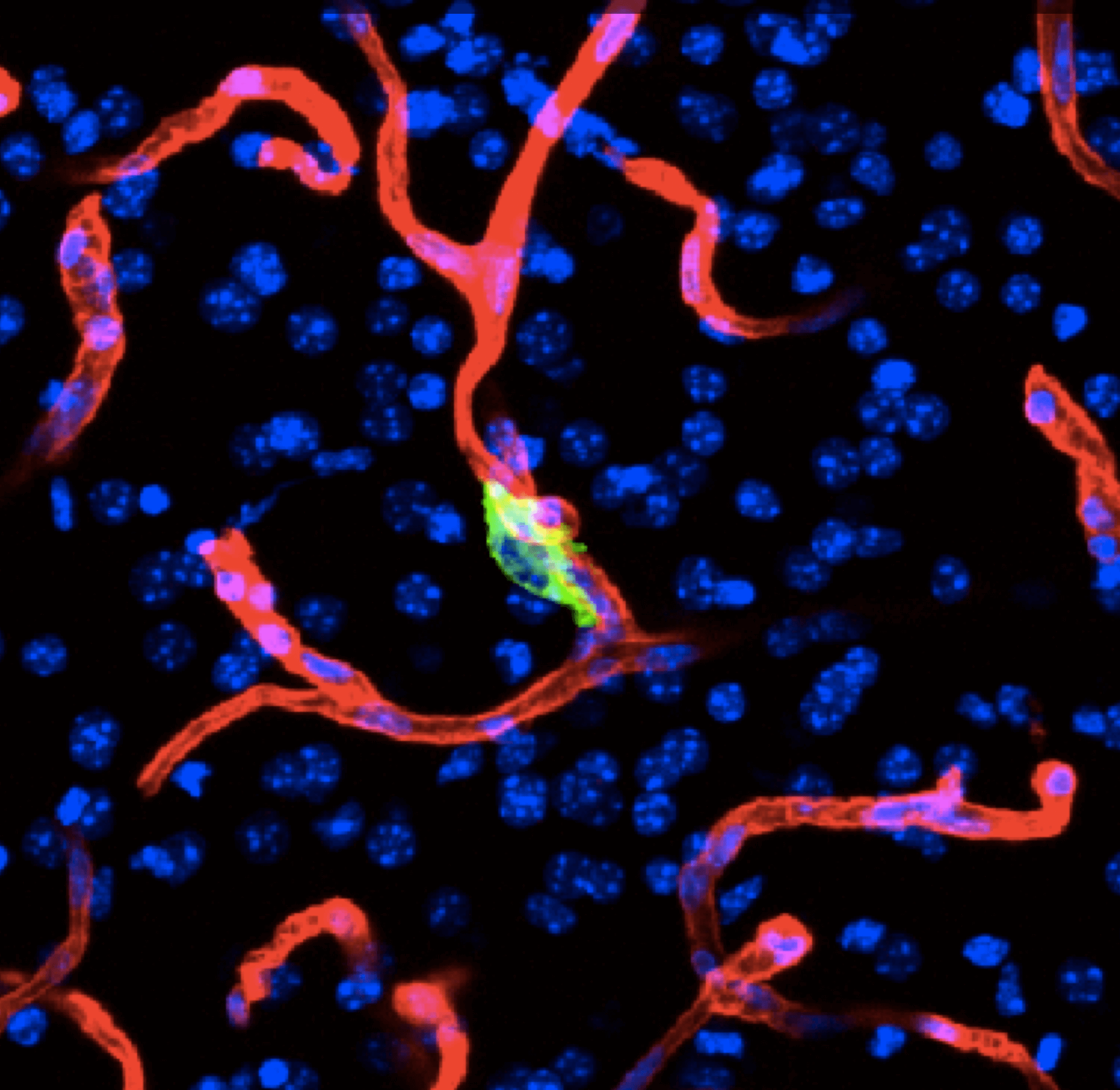
Metastasis-initiating cells become specialized at colonizing one or multiple organs, giving rise to organ tropisms that are characteristic of each type of cancer. The lab has identified a series of mediators of organ-specific metastasis and is currently focusing on the specific case of brain metastasis. Brain metastasis is highly lethal. Its incidence is ten-fold higher than that of all other brain tumor types combined. The lab has created a panel of mouse models of brain metastasis from lung cancer and breast cancer. We are using these models to identify clinically relevant mediators of brain metastasis. In recent work we demonstrated the existence of intimate interactions between brain metastatic cells and astrocytes through GAP junctions that engage astrocytes in support of tumor growth. We are combining these models and human metastasis samples to identify targetable tumor-stroma interactions that support metastasis. We interrogate the crosstalk between metastasis and the main cellular components of the brain (astrocytes, microglia, endothelia and neurons) using tumor stroma capture techniques, imaging, single-cell analytics, and in situ transcriptomics. We collaborate with system biologists, imaging experts and physicians. Clinical trials targeting the brain metastasis micro environment have been initiated.