Telomere crisis, which occurs during tumorigenesis when short telomeres become dysfunctional, is hypothesized to be a major source of the instability observed in cancer genomes (Figure 1). Telomere crisis is characterized by telomere fusions, genomic instability, and cell death and gives rise to aneuploidy, non-reciprocal translocations, whole-genome duplication, chromothripsis and kataegis. Evidence of prior telomere crisis has been detected in chronic lymphocytic leukemia (CLL), breast cancer, colorectal adenomas, and a variety of other solid tumors and is associated with a poor prognosis in CLL and breast cancer. Ultimately, telomerase reactivation provides a mechanism for cells to escape this telomere dysfunction. We utilize live-cell imaging, genetic approaches, and an established sequencing pipeline to clarify the causes of alteration to the cancer genome and to investigate the impact of the cytosolic DNA species that accumulate during telomere crisis.
Outcome and mechanisms of telomere crisis-derived genome instability
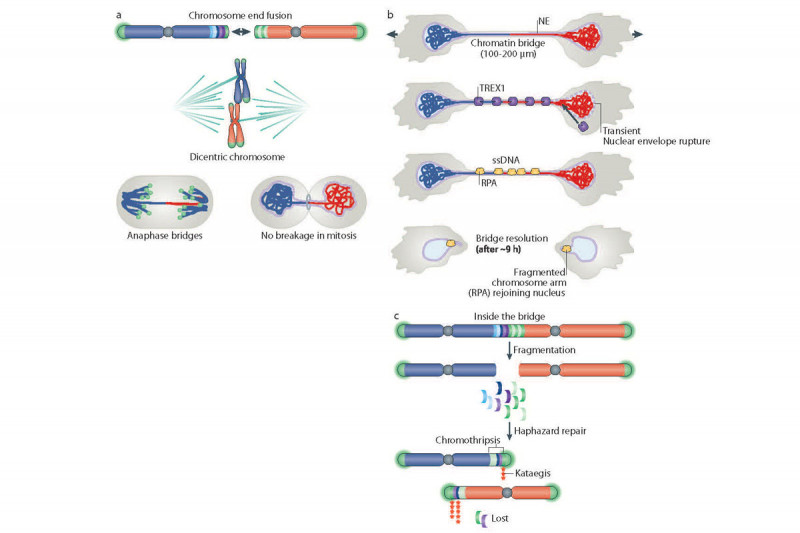
Telomere crisis threatens genome stability through the formation of dicentric chromosomes. Dicentric chromosomes, or chromosomes with two centromeres, can be destabilized during mitosis by making attachments to opposite spindle poles. Preliminary observations of apparent dicentric chromosome breakage during anaphase initiated the widely held view that dicentrics cannot survive mitosis intact. Given relatively weak mitotic spindle pulling forces (~1nN) and strong chromosome tensile strength (>100 nN), we hypothesized that the mitotic spindle could not break dicentric chromosomes. To test this we have developed a conditional system to mimic telomere crisis and address outstanding questions regarding the resolution of dicentric chromosomes and the genomic legacy of telomere crisis. Using this model, we have shown that dicentrics survive mitosis intact (Figure 2) and develop into chromatin bridges that connect daughter cells into the next cell cycle (Figure 3). These bridges are attacked by the cytoplasmic nuclease, TREX1, which gains access to the DNA during nuclear envelope rupturing events induced by chromatin bridges (Figure 4). Cells that survive telomere crisis display frequent chromothripsis (chromosome shattering) and kataegis (clustered, strand-coordinated mutations), suggesting that the DNA damage that precedes these catastrophic events is compartmentalized to chromatin bridges (Figure 5).
Current work in the lab is focused on obtaining mechanistic insight into how telomere crisis impacts genome stability. A major open question is how dicentric chromosomes can trigger the accumulation of clusters of chromosome rearrangements and rearrangement-proximal mutations. One avenue under investigation by the lab is the attack of genomic DNA by cytosolic enzymes such as TREX1 and the APOBEC family of cytidine deaminases. The APOBECs are a family of enzymes that can deaminate cytosine residues to uracil and therefore act as DNA mutators, thus contributing to one of the most prevalent mutational signatures in cancer. Many APOBECs are active in the cytoplasm, where they restrict RNA and DNA virus infection, thereby contributing to an innate retroviral defense.
A second major area of research in the lab centers on the unexplored consequences of nuclear envelope rupture, which is a frequent event during telomere crisis and cell migration. Nuclear envelope rupturing promotes DNA damage and genome instability, potentially acting as a potent mutagen during cancer cell metastasis. We seek to determine mechanisms of how nuclear envelope rupturing events compromise genomic integrity.
Finally, we are intrigued by the possibility that the cytosolic DNA species that accumulate during telomere crisis may be detected by the surveillance machinery of the innate immune system and induce downstream signaling pathways with potential consequences for cancer development.