The zebrafish is a relative newcomer to the cancer field, which has necessitated that we develop a range of new technologies. Some key highlights:
Novel transgenic technologies
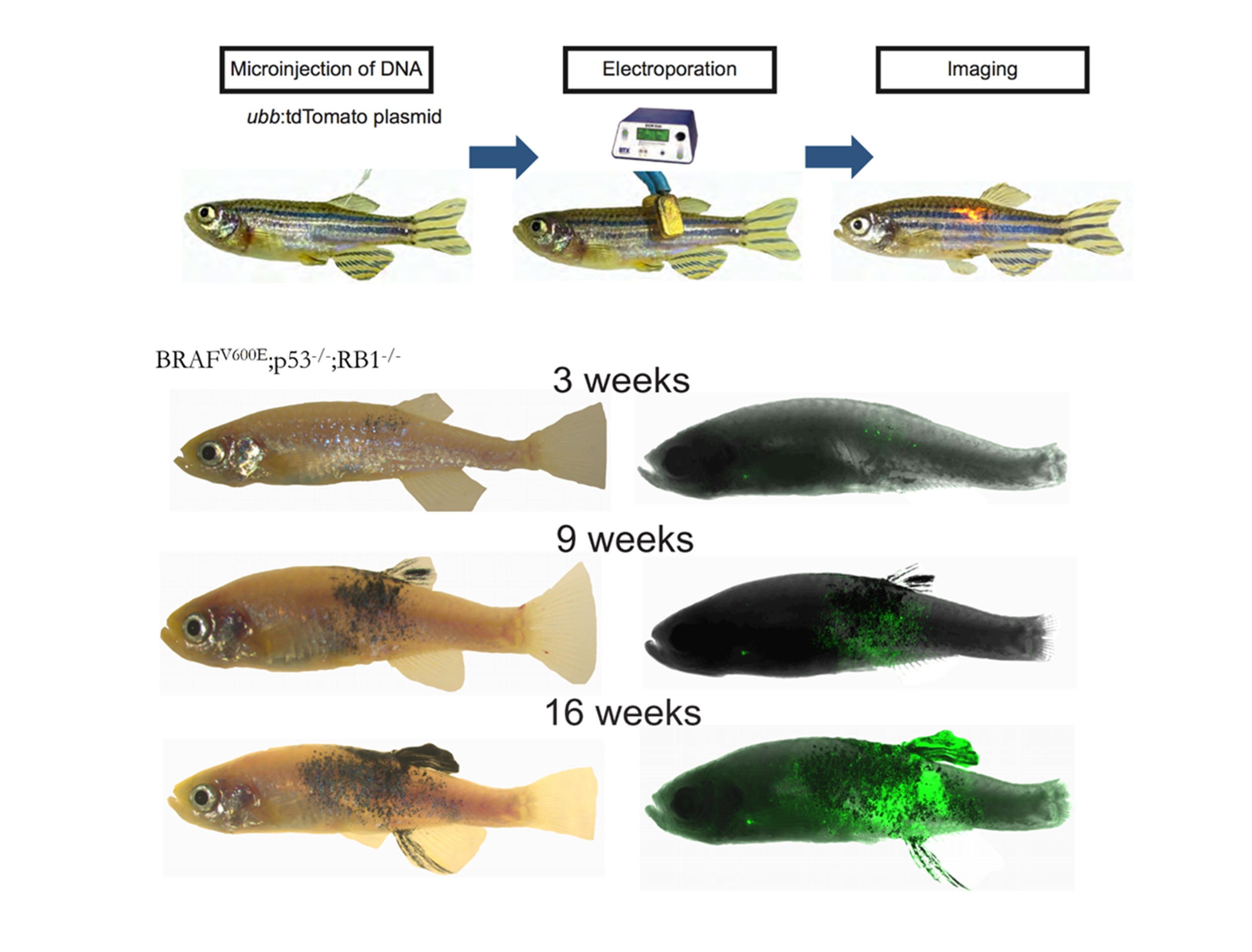
We have developed a new somatic transgenesis technique called TEAZ (Transgene Electroporation in Adult Zebrafish) that allows for rapid and precise delivery of DNA cargo to cells of interest (Callahan, et al, Disease Models and Mechanisms 2018). This method entirely bypasses germline transgenesis and allows for widespread cDNA/CRISPR screening of both tumor and microenvironmental genes in melanoma progression.
Transplantable zebrafish cell lines
As a complement to what can be achieved with TEAZ, we have also established several zebrafish specific melanoma cell lines such as the ZMEL1 line (Heilmann, et al, Cancer Research 2015). These can be rapidly manipulated ex vivo and then transplanted into the transparent casper zebrafish. These tumors rapidly give rise to widespread metastatic disease.
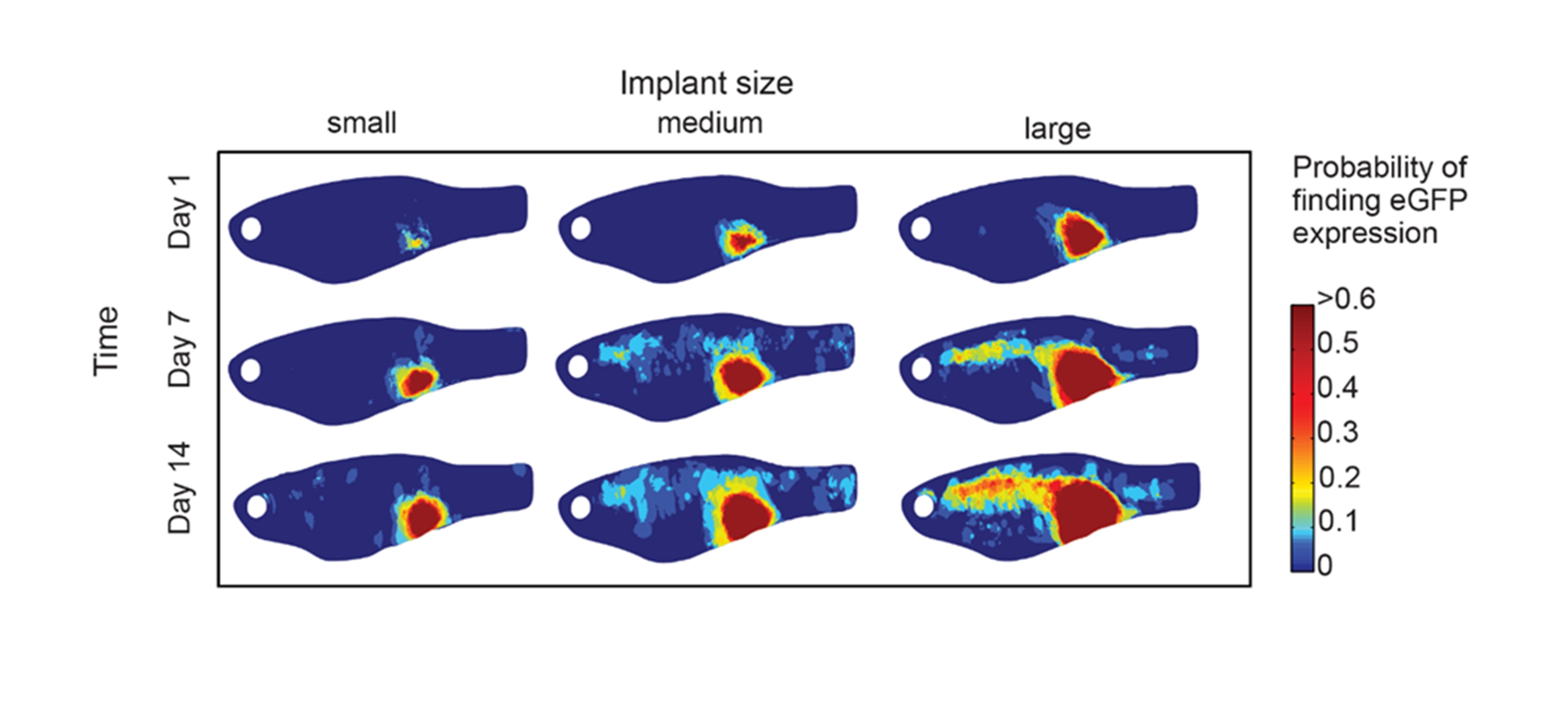
Tracking of ZMEL1-GFP melanoma cells as they metastasize in the transparent casper strain of zebrafish.
Advances in zebrafish imaging
Given the optical transparency of casper, we have developed several pipelines that allow us to precisely quantify the rate and extent of metastatic progression in the fish. This imaging ranges from single cell to whole animal, something that is difficult to achieve in other cancer model systems.
Chemical genetic screening
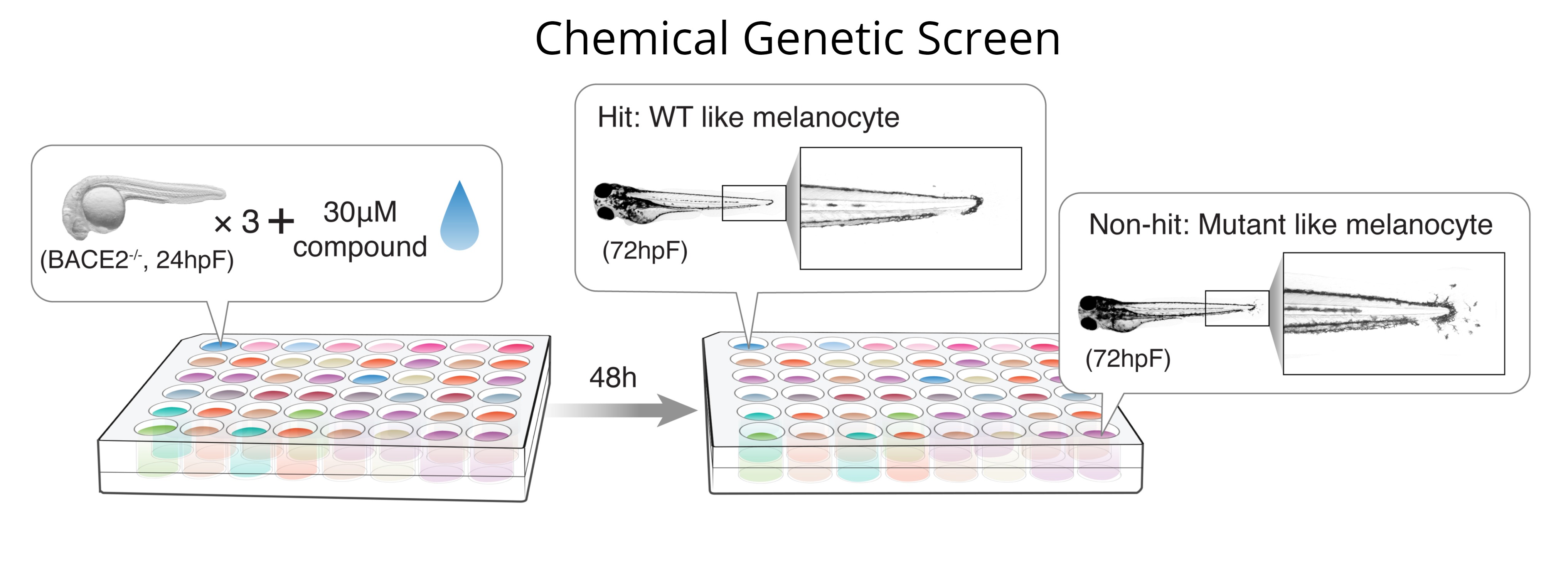
A unique feature of the zebrafish is that it is amenable to chemical genetic screens, in which small molecules can be directly added to the fish water and the fish then analyzed for phenotypes of interest (Kaufman, et al, Nature Protocols 2009). We have used this method to identify suppressors of the embryonic neural crest (White, et al, Nature 2011) and to find modifiers of melanocyte cell state via the sheddase BACE2 (Zhang, et al, Developmental Cell 2018). These screens can be adapted to 96-well format and automated imaging, allowing for a combination of high-throughput/high-content screening.
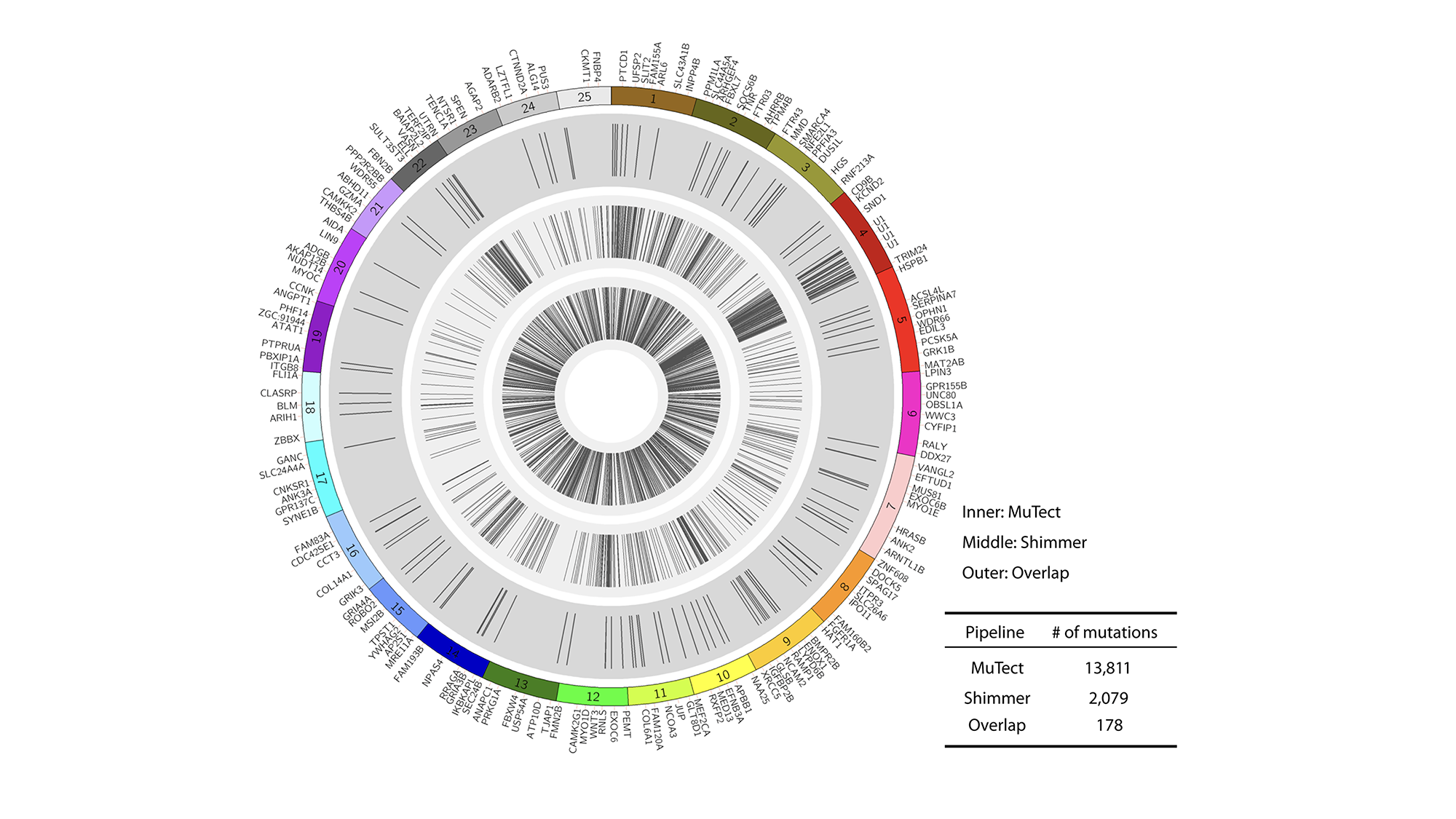
Genomic analysis of zebrafish tumors
While advances in genomic analysis of human cancers have moved rapidly, fewer tools exist to understand how zebrafish cancers are altered at the DNA or RNA levels. We performed the first large-scale exome, whole-genome and RNA-seq analyses of the zebrafish melanomas (Yen, et al Genome Biology 2013 and Kansler, et al, BMC Genomics 2017). This has revealed strong conservation of function between the zebrafish melanomas and human melanomas, highlighting the relevance of our model to the human disease.