Diversity-Oriented Synthesis and Rational Drug Design for Chemical Biology and Drug Discovery Research
Research in the Tan Lab focuses on synthetic organic chemistry and its applications to current challenges in chemical biology and drug discovery.
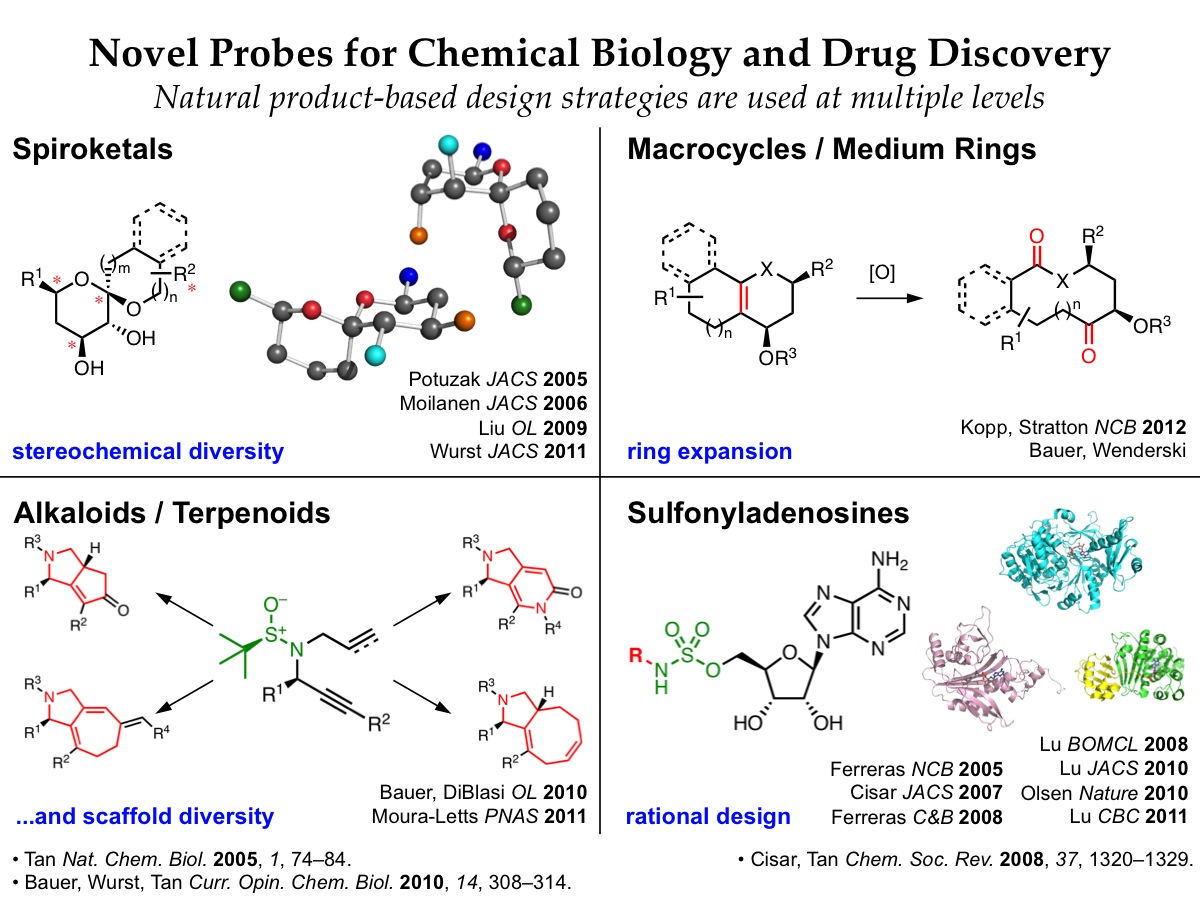
In the area of rational drug design, we are developing small molecule inhibitors that target adenylation enzymes, a mechanistic superfamily that is involved in a wide range of biological processes implicated in human diseases. We use mechanistic and structural information about individual targets of interest to design small molecule inhibitors, leveraging a natural product-based sulfonyladenosine design platform. Among these are novel antibiotics that inhibit bacterial biosynthesis of siderophores, menaquinone, and phenolic glycolipids. Semisynthetic protein-based inhibitors of eukaryotic E1 activating enzymes have also revealed striking enzyme remodeling during the conjugation of ubiquitin and other ubiquitin-like modifers to target proteins. We leverage synergistic multidisciplinary collaborations with biologists in our Tri-Institutional Research program and at other institutions to pursue these efforts.
We are also engaged in a complementary program in diversity-oriented synthesis, in which we are developing new synthetic routes and strategies to access small molecules that contain key structural motifs found in biologically active natural products. These efforts provide exciting opportunities to develop new synthetic methodologies and fundamental insights into chemical reactivity. Efficient, flexible synthetic routes are used to generate natural product-based libraries that access regions of chemical space not addressed by conventional drug-like libraries. These natural product-based libraries are being screened against a wide range of promising new therapeutic targets through collaborations with other labs in the Tri-Institutional Research Program, the Broad Institute, and the NIH Molecular Libraries Initiative.
More information about individual projects is available below.
Science Spotlight lecture: Derek Tan, PhD
Derek presented a seminar in the MSK Science Spotlight online seminar series on June 15, 2020. The presentation, entitled “A Drug Discovery Journey from Tuberculosis to Cancer”, highlights some work from the lab’s longstanding program in tuberculosis drug discovery, and describes how this work led to more recent applications of these molecules in a novel cellular therapy platform that combines the immunologic effects of CAR-T cells with the anticancer activity of small-molecule drugs that are generated locally at tumor sites.
Derek’s CDD Webinar on Antibiotic Discovery on Mar 22, 2018 is available online. This seminar and discussion with Prof. Helen Zgurskaya of the University of Oklahoma and Dr. Brad Sherborne of Merck & Co. gives insights into the challenges of penetrating Gram-negative bacteria with small-molecule antibiotics and current approaches to tackling this important problem.
[ Video and Slide Presentation ]
Derek has been featured in Science Forward, a video series produced by the Macaulay Honors College at the City University of New York (CUNY). In the video on “Drug Development and Discovery”, Derek talks about the wide range of skills and approaches chemical biologists use to design and synthesize new potential drugs. Viewers can catch a glimpse of the Tan lab as well as TPCB student Michaelyn Lux in action. In “Scientific Uncertainty”, Derek is quoted on the exciting role of uncertainty in our research.
[ Science Forward: Drug Development and Discovery | Scientific Uncertainty ]
Derek’s talk at the National Academy of Sciences U.S. Kavli Frontiers of Science Symposium on Nov 12, 2009 is available online. This 30-minute Flash presentation gives an overview of our research for a general scientific audience.
[ Program | Abstract (PDF) | Video | Vimeo Mirror ]
Key Review Articles
-
Stereocontrolled synthesis of spiroketals: An engine for chemical and biological discovery
Verano, A. L.; Tan, D. S.* Isr. J. Chem. 2017, 57, 279–291.
[ Abstract | PubMed | PMC ] Cheminformatic comparison of approved drugs from natural product versus synthetic origins.
Stratton, C. F.; Newman, D. J.; Tan, D. S.* Bioorg. Med. Chem. Lett. 2015, 25, 4802–4807.
[ Abstract | PubMed | PMC ]Principal component analysis as a tool for library design: A case study investigating natural products, brand-name drugs, natural product-like libraries, and drug-like libraries.
Wenderski, T. A.; Stratton, C. F.; Bauer, R. A.; Kopp, F.; Tan, D. S.* Methods Mol. Biol. 2015, 1263, 225–242.
[ Abstract | PubMed | PMC ]Expanding the range of ’druggable’ targets with natural product-based libraries: An academic perspective.
Bauer, R. A.; Wurst, J. M.; Tan, D. S.* Curr. Opin. Chem. Biol. 2010, 14, 308–314.
[ Abstract | PubMed | PMC ]Small molecule inhibition of microbial natural product biosynthesis – An emerging antibiotic strategy.
Cisar, J. S.; Tan, D. S.* Chem. Soc. Rev. 2008, 37, 1320–1329.
[ Abstract | PubMed | PMC ]Diversity-oriented synthesis: Exploring the intersections between chemistry and biology.
Tan, D. S.* Nat. Chem. Biol. 2005, 1, 74–84.
[ Abstract | PubMed ]
Research Projects
-
Rational Drug Design of Adenylation Enzyme Inhibitors
Adenylation enzymes mediate a variety of important bacterial and eukaryotic pathways. We are using rational drug design to develop sulfonyladenosine-based inhibitors of adenylation enzymes as new antibiotics and chemical probes. -
Ring Expansion Approaches to Macrocycle Synthesis
Macrocycles are an attractive class of molecules for use in biological screening. While smaller molecules have been used to bind in enzyme active sites and other protein pockets, larger molecules such as macrocycles may be useful for affecting protein-protein interactions involving large surface areas… . -
Stereoselective Diversity-Oriented Synthesis of Spiroketals
Spiroketals are privileged substructures found in numerous natural products with diverse biological activities. We are developing stereoselective diversity-oriented synthesis routes to provide spiroketal libraries with comprehensive stereochemical diversity for screening against a wide range of biological targets. -
Diversity-Oriented Synthesis of Multiscaffold Alkaloid Libraries
An amazing range of rigid, densely functionalized polycyclic compounds are formed from simple precursors during the biosynthesis of alkaloids and terpenoids. To access such structures, we are using a related diversity-oriented synthesis strategy to generate multiscaffold polycyclic libraries from a small set of simple precursors.