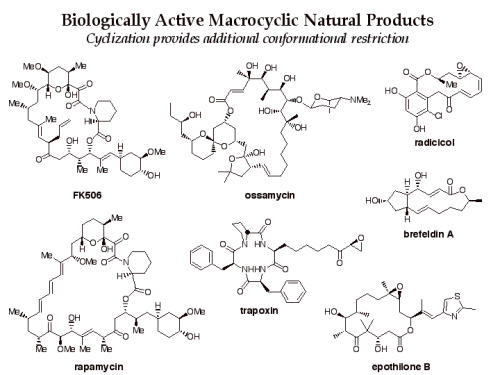
Macrocycles are an attractive class of molecules for use in biological screening. While smaller molecules have been used to bind in enzyme active sites and other protein pockets, larger molecules, such as macrocycles, may be useful for affecting protein-protein interactions involving large surface areas.
Furthermore, the potential diversity of overall 3D structures present in a library of macrocycles is enhanced by the fact that conformation can be altered by strategic changes in substituent patterns and stereochemistry.
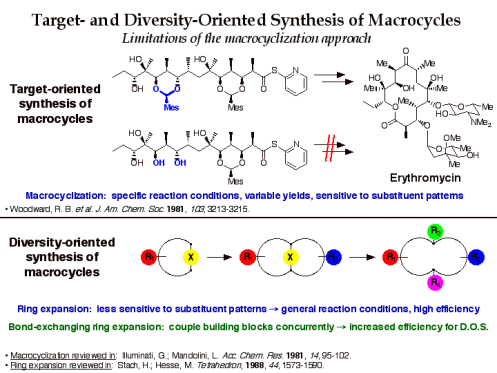
Target-oriented synthesis of macrocyclic natural products is dominated by macrocyclization strategies that often require ad hoc development of reaction conditions, proceed in moderate yields, and are sensitive to ring substituent patterns. Because diversity-oriented synthesis requires efficient reactions that are general for a wide variety of substrates, ring expansion is an attractive alternative strategy for the synthesis of macrocycle and medium ring libraries.
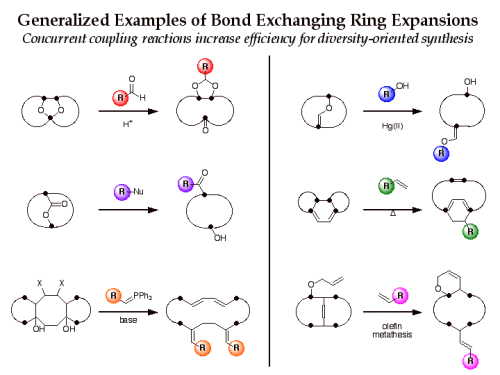
The efficiency of this approach for diversity-oriented syntheses can be increased if the bond-cleaving ring expansion is performed in conjunction with bond-forming building block coupling in a “bond-exchanging” reaction. Examples of bond-exchanging reactions include nucleophilic substitution, carbonyl addition-elimination, transetherification, olefin metathesis, and tandem cycloaddition-cycloreversion. We are exploring a number of bond-exchanging ring expansion routes to macrocycles for application to diversity-oriented synthesis.
Publications
Principal component analysis as a tool for library design: A case study investigating natural products, brand-name drugs, natural product-like libraries, and drug-like libraries.
Wenderski, T. A.; Stratton, C. F.; Bauer, R. A.; Kopp, F.; Tan, D. S.* Methods Mol. Biol. 2015, 1263, 225–242.
[ Abstract | PubMed | PMC ]