Cancer Biology & Genetics Program
The Joan Massagué Lab
Research

The Massagué lab investigates metastasis stem cells and their stromal niches throughout the metastatic cascade, with a growing interest in the dormant phase of metastasis. We are defining the phenotypic plasticity and evolution of metastatic cell populations from dormancy to outbreak, identifying drug targets, and enabling clinical trials to treat metastasis. We entered this area on realizing the central role of TGF-β and its interplay with oncogenic signals in metastasis. We collaborate with biologists, data scientists, and clinicians to leverage these findings for clinical benefit.
Stem Cell Signaling, Growth, Control, and Cancer Metastasis
TGF-β signaling in development and disease
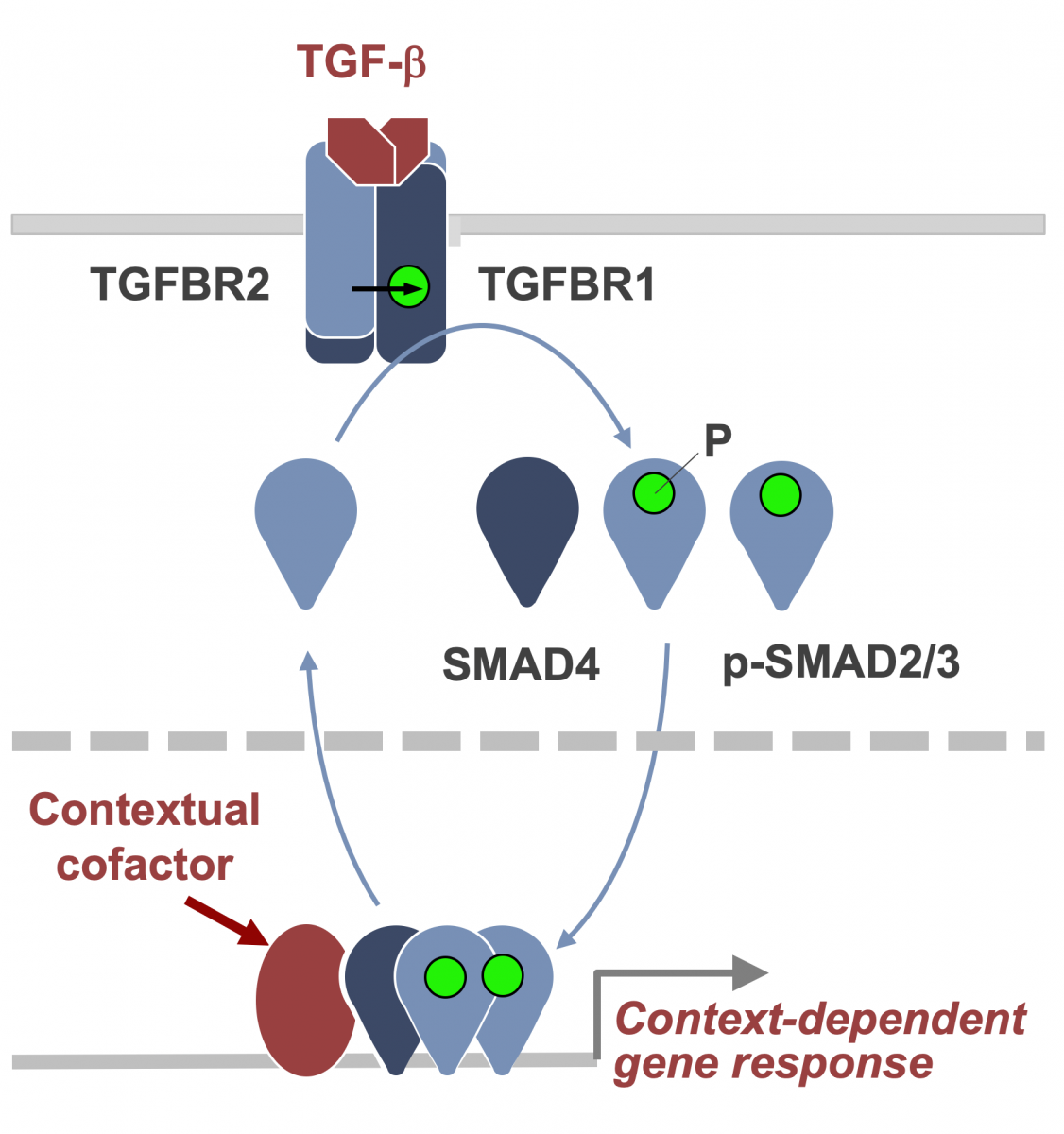
The TGF-β regulatory system plays crucial roles in the preservation of organismal integrity by simultaneously regulating different cell types and cellular functions. TGF-β signaling controls embryo development, tissue homeostasis, and injury repair through coordinated effects on cell proliferation, phenotypic plasticity, migration, metabolic adaptation, and immune surveillance of multiple cell types in shared ecosystems. Defects of TGF-β signaling disrupt immune tolerance, promote inflammation, underlie the pathogenesis of fibrosis and cancer, and contribute to the resistance of these diseases to treatment. Having elucidated the TGF-β signaling pathway, we are investigating how cells interpret TGF-β signals depending on the presence of context-dependent SMAD cofactors and chromatin configurations. Recent work uncovered a major interface between the TGF-β and RAS-MAPK pathways. TGF-β and RAS, signaling through SMAD and RAS-responsive element-binding protein 1 (RREB1) respectively, jointly activate a multi-arm regulatory program in epithelial progenitors and carcinoma cells (Su et al Nature 2020; Lee et al Cell 2024). RREB1 localizes to acetylated histone H4 marks in histone H2A.Z-loaded nucleosomes in cell plasticity genes, priming these enhancers for activation by a TGF-β activated SMAD4-INO80 nucleosome remodeling complex. These findings illuminate the operation of a bifunctional program that promotes metastatic outgrowth.
Phenotypic plasticity regulation
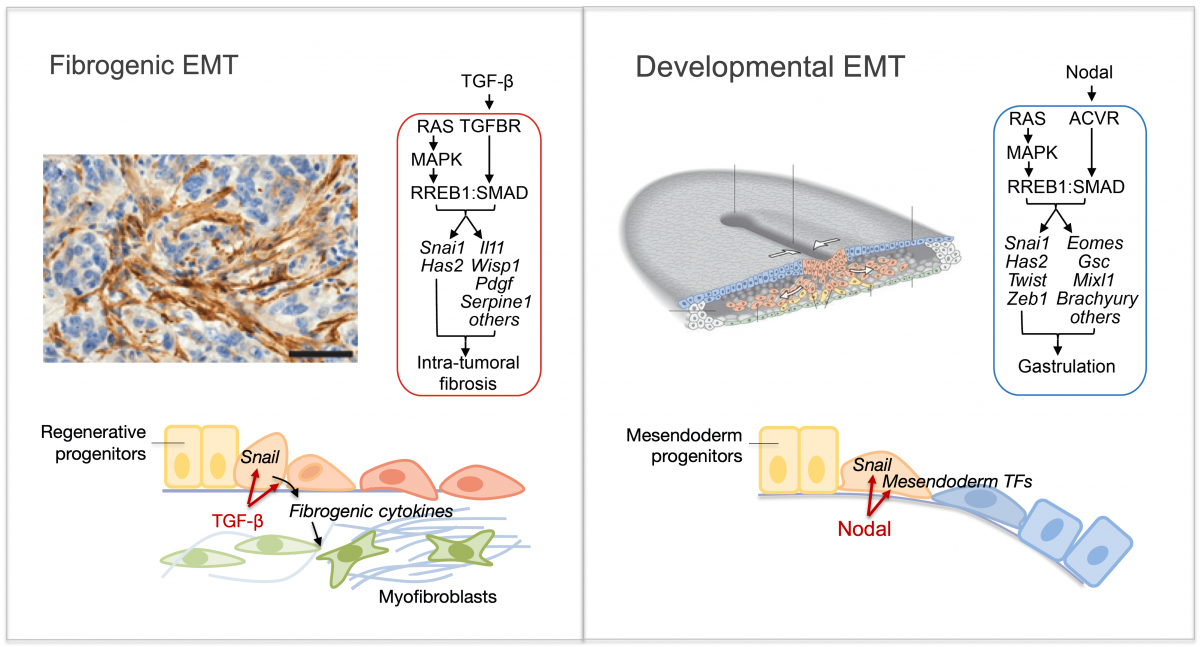
Epithelial-to-mesenchymal transitions (EMTs) are phenotypic plasticity processes that confer migratory and invasive properties to epithelial cells during development, wound-healing, and cancer. TGF-β is a potent inducer of EMTs implicated in liver disease, pulmonary fibrosis, and carcinoma metastasis. Lung adenocarcinoma (LUAD) cells undergo a TGF-β-dependent EMT associated with fibroblast activation and extracellular matrix remodeling. RAS-activated RREB1 primes enhancers of EMT and fibrogenic genes for activation by chromatin remodeling complexes that the TGF-β/SMAD pathway recruits to these enhancers. Both the EMT arm and the fibrogenic arm of this response program are essential for pulmonary metastasis in LUAD models. Inhibiting RREB1 disables this pro-metastatic process.
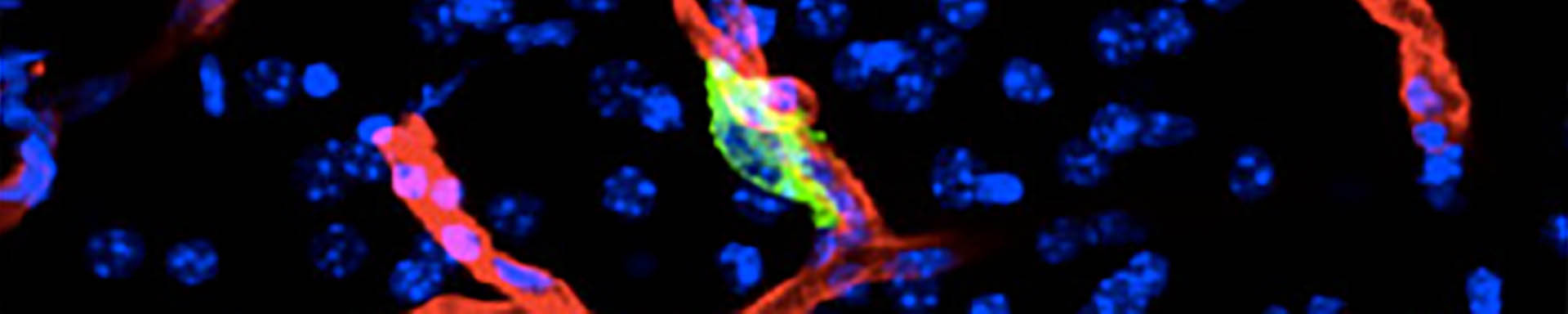
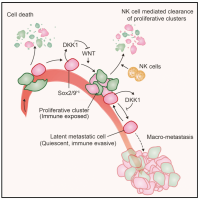
In primitive, stem-like SOX2+ LUAD progenitors, TGF-β induces growth arrest accompanied by a full EMT response that subsequently transitions into an atypical mesenchymal state of round morphology and lacking actin stress fibers. TGF-β drives this long-term transition by inducing the expression of gelsolin, which converts a stress fiber-rich mesenchymal phenotype into a cortical actin-rich spheroidal state of low biomechanical stiffness to protect metastatic stem cells from killing by CD8 T cells and NK cells. Thus, LUAD primitive progenitors undergo an atypical EMT as part of a strategy to evade immune-mediated elimination (Wang et al Nature Cancer 2026). We are building on these insights to gain a better understanding of epithelial plasticity regulation by TGF-β in development, fibrosis and metastasis.
Metastasis initiating cells
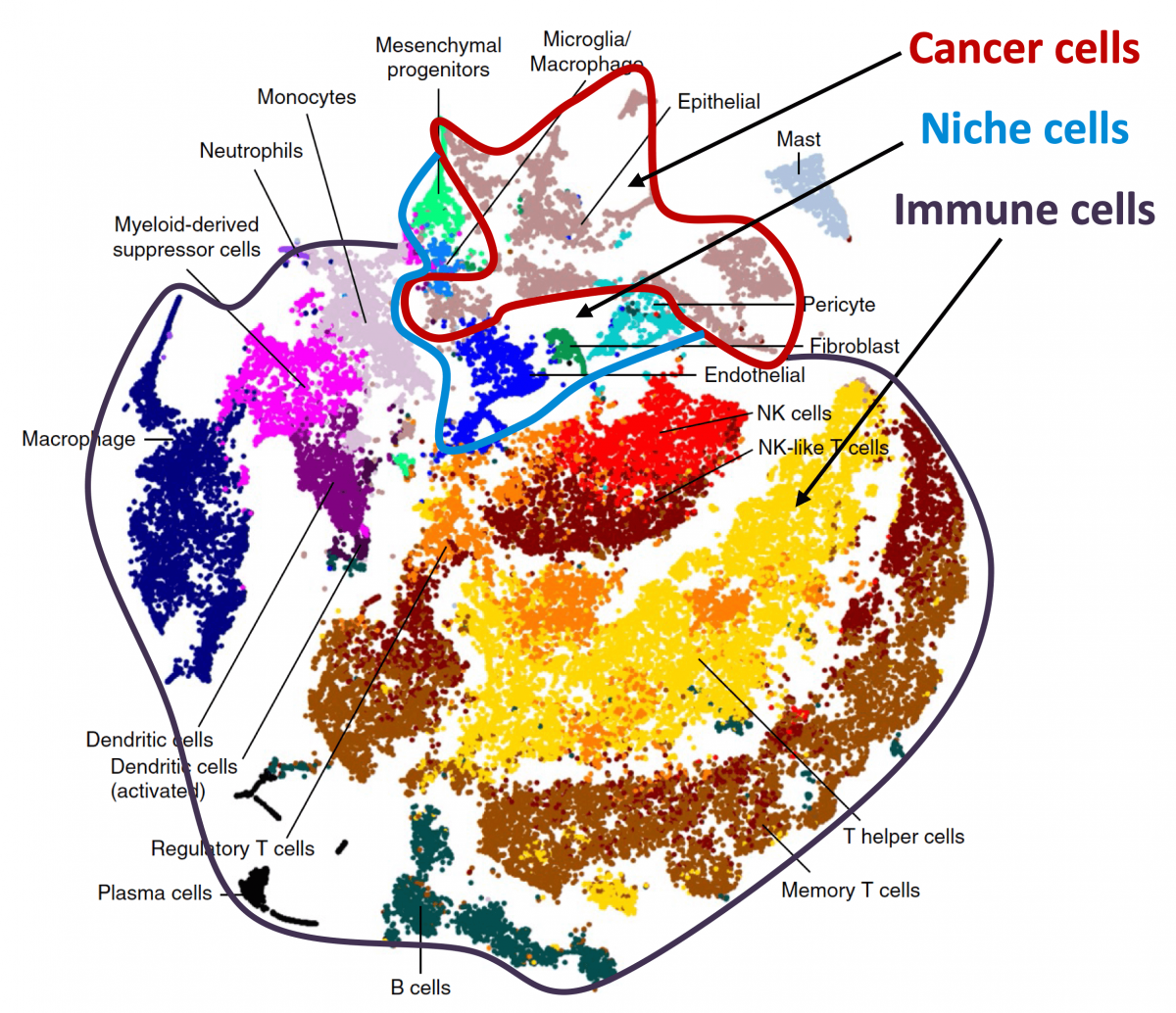
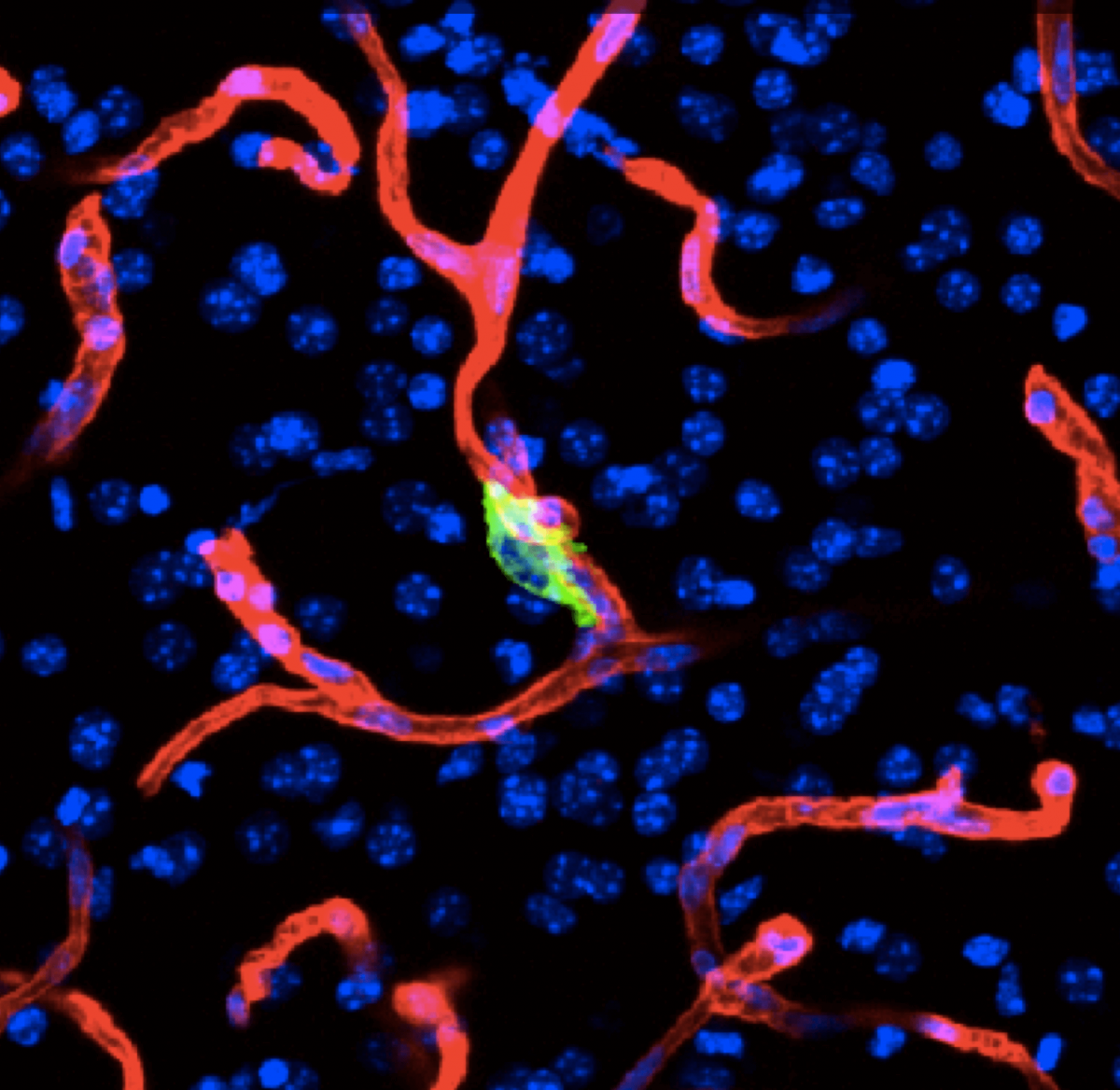
Tissue homeostasis is maintained by stem cells, whereas damaged tissues are repaired by facultative progenitors that are activated upon injury. Developmental processes underlying normal tissue regeneration also operate in metastasis. Metastasis remains the main cause of death from cancer. The persistence and lethal relapse of disseminated cancer is driven by stem-like metastasis initiating cells. We are interested in understanding how the metastasis initiating phenotype emerges during tumor progression. The cell adhesion molecule L1CAM is a marker of the metastasis initiating phenotype in breast, lung, colorectal and renal carcinomas. L1CAM expression is silent in normal epithelial tissues but is active and required during wound healing. Metastasis initiating cells require L1CAM for colonization of multiple organs (brain, lung, lives and bone). Disseminated cancer cells use L1CAM to spread on blood capillaries and activate mechano-transduction transcription factors for tumor outgrowth. Using patient tumor tissues, organoids, mouse models, lineage tracing, advanced imaging, and single cell analytics, the lab is investigating the nature of metastasis initiating cells and their stromal niches and evaluating L1CAM and related molecules as therapeutic targets.
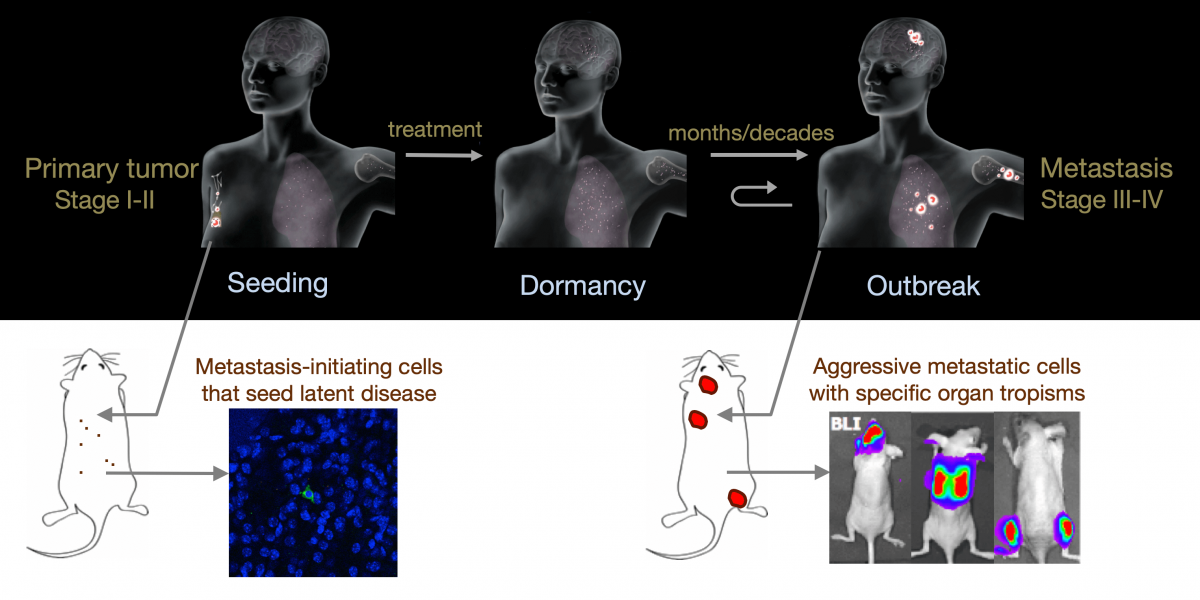
From metastatic dormancy to outbreak
Metastasis is a complex, multiorgan, and often fatal process (accounting for 90% of cancer-related deaths). Cancer cells that disseminate from a tumor to distant sites enter a period of dormancy that may persist from months to decades before giving rise to detectable metastasis. Adjuvant therapy treatments seek to prevent overt metastasis by eliminating residual malignant cells during this dormancy period. Efforts to improve adjuvant therapy are hindered by an insufficient understanding of the molecular mechanisms that preserve the long-term viability of dormant metastatic cells. Identifying these mechanisms is needed to improve treatments and prevent relapse. Using mouse models that the lab developed, we are investigating the evolution of SOX2+ metastasis stem cells that enter a dormant phase after infiltrating target organs, remain viable under immune surveillance, and eventually reinitiate tumor growth. This work revealed roles of TGF-β as a driver of immune evasive metastatic dormancy and reveal vulnerabilities of metastatic cells. We uncovered key roles of STING (Hu et al Nature 2023) and L1CAM in this process (Park et al bioRxiv 2025) that can be exploited for the eradication of residual disease.
Organ-specific metastasis
During metastasis, disseminated cancer cells undergo profound, tissue-specific reprogramming that reshapes their phenotype, metabolism, and therapeutic responses, giving rise to organ adaptations and organ tropisms characteristic of each type of cancer. The lab has identified a series of mediators of organ-specific metastasis and is currently focusing on the specific case of brain metastasis. Brain metastasis is highly lethal. Its incidence is ten-fold higher than that of all other brain tumor types combined. We created mouse models of brain metastasis from lung cancer and breast cancer and are using these models to identify relevant mediators of brain metastasis. In recent work we demonstrated that distinct tumor architectures and microenvironments for the initiation of metastasis in the brain (Gan et al Cancer Cell 2024). This work argues for an precision oncology framework that integrates these organ site-specific programs into treatment design.
Featured News
Publications Highlights
Wang Z, Elbanna Y, Godet I, Peters L, Lampe G, Chen Y, Xavier J, Huse M, Massagué J. TGF-β induces an atypical EMT to evade immune mechanosurveillance in lung adenocarcinoma dormant metastasis. Nature Cancer. (2026) Jan 5; DOI: 10.1038/s43018-025-01094-y [PMID: ] [PMCID: ]
Kawasaki K, Salehi S, Zhan YA, Chen K, Lee JH, Salataj E, Zhong H, Manoj P, Kinyua D, Mello BP, Sridhar H, Tischfield SE, Linkov I, Ceglia N, Zatzman M, Havasov E, Shah NJ, Meng F, Loomis B, Bhanot UK, Redin E, de Stanchina E, Hamard PJ, Koche RP, McPherson A, Quintanal-Villalonga A, Shah SP, Massagué J* and Rudin CM* (*corresponding). FOXA2 promotes metastatic competence in small cell lung cancer. Nature Commun. 16:4865, (2025) [PMID: 40419484] [PMC1210783] https://doi.org/10.1038/s41467-025-60141-5
Lee JH, Sánchez-Rivera FJ, He L, Basnet H, Chen F, Spina E, Li L, Torner C, Chan JE, Yarlagadda DVK, Park JS, Sussman C, Rudin CM, Lowe SW, Tammela T, Macias MJ, Koche RP, Massagué J. TGF-β and RAS jointly unmask primed enhancers to drive metastasis. Cell (2024) Oct 31;187(22):6182-6199.e29. DOI: 10.1016/j.cell.2024.08.014 PMID: 39243762 PMCID: PMC12035776
People

Joan Massagué, PhD
Director, Sloan Kettering Institute; Member, Cancer Biology & Genetics Program; Marie-Josée and Henry R. Kravis Chair
- Joan Massagué studies the control of stem cell growth and phenotype in tumor progression, metastasis, and response to therapy.
- PhD, University of Barcelona
- [email protected]
- Email Address
- 646-888-2044
- Office Phone
Members
















- 646-888-2063
- Lab Phone

- BS, Peking University (Physics)
- PhD, Harvard University (Systems Biology)
- BS, Massachusetts Institute of Technology
- BS, Bilkent Universitesi
- Cornell/Sloan Kettering Graduate School

- Binghamton University
- Cornell/Sloan Kettering Graduate School
- Cornell/Sloan Kettering Graduate School
- U. Med Sciences Beijing
- Roswell Park Cancer Institute, NY

- University of Barcelona
- Rockefeller/Cornell/Sloan Kettering
- Rockefeller University
- University of Turku, Finland
- University of Rome
- Cornell/Sloan Kettering Graduate School
- Duke University

- Cornell University, Ithaca
- University of Helsinki, Finland
- Cornell/Sloan Kettering Graduate School
- Rockefeller/Cornell/Sloan Kettering
- Wesleyan University
- University of Texas, Austin
- University of Rochester
- Medical University of Graz, Austria
- ISREC
- Rockefeller Cornell/Sloan Kettering
- 1987, University of Santiago, Spain
- Cornell/Sloan Kettering Graduate School
- Uppsala University
- University of Barcelona
- University of Barcelona, Spain

- McMaster University, Ontario, Canada
- Harvard Medical School
- Institute of Neuroscience, Alicante, Spain
- University of Helsinki, Finland
- Cornell/Sloan Kettering Graduate School
- University of York
- University of Toronto
- New York University, New York
- London Research Institute
- Columbia University, New York
- Tsinghua University


















































































































Achievements
- Chief Scientific Officer, MSKCC (2023–2025)
- Director, Sloan Kettering Institute (2014–)
- HHMI Scientific Review Board (2014–2025)
- HHMI Investigator (1990-2013)
- Fellow, American Association for Cancer Research (AACR) Academy (2016)
- Pezcoller Foundation-AACR International Award for Cancer Research (2016)
- Charles Rodolphe Brupbacher Prize for Cancer Research (2015)
- National Prize for Research in Biology, Spain (2014)
- American Italian Cancer Foundation Price (2013)
- Prize in Cancer Research, Robert J. and Claire Pasarow Foundation (2011)
- Frontiers Prize in Biomedicine, BBVA Foundation (2008)
- Vilcek Prize, Vilcek Foundation (2006)
- Member, Institute of Medicine (2006)
- Award in Science and Technology, Prince Asturius Foundation (2004)
- Member, National Academy of Sciences (2000)
- Member, American Academy of Arts and Sciences (1999)
- Investigator, Howard Hughes Medical Institute (1990)
- Elucidation of the TGF-beta pathway
Lab News & Events
Open Positions
To learn more about available postdoctoral opportunities, please visit our Career Center
To learn more about compensation and benefits for postdoctoral researchers at MSK, please visit Resources for Postdocs
Post Doctoral Position
Get in Touch
-
Lab Head Email
-
Office Phone
-
Lab Phone
Disclosures
Members of the MSK Community often work with pharmaceutical, device, biotechnology, and life sciences companies, and other organizations outside of MSK, to find safe and effective cancer treatments, to improve patient care, and to educate the health care community. These activities outside of MSK further our mission, provide productive collaborations, and promote the practical application of scientific discoveries.
MSK requires doctors, faculty members, and leaders to report (“disclose”) the relationships and financial interests they have with external entities. As a commitment to transparency with our community, we make that information available to the public. Not all disclosed interests and relationships present conflicts of interest. MSK reviews all disclosed interests and relationships to assess whether a conflict of interest exists and whether formal COI management is needed.
Joan Massagué discloses the following relationships and financial interests:
-
Institute for Research in Biomedicine Barcelona
Professional Services and Activities
-
Scholar Rock
Equity
The information published here is a complement to other publicly reported data and is for a specific annual disclosure period. There may be differences between information on this and other public sites as a result of different reporting periods and/or the various ways relationships and financial interests are categorized by organizations that publish such data.
This page and data include information for a specific MSK annual disclosure period (January 1, 2024 through disclosure submission in spring 2025). This data reflects interests that may or may not still exist. This data is updated annually.
Learn more about MSK’s COI policies here. For questions regarding MSK’s COI-related policies and procedures, email MSK’s Compliance Office at [email protected].


