PU-CLASS (PURINE-SCAFFOLD HSP90 INHIBITORS)
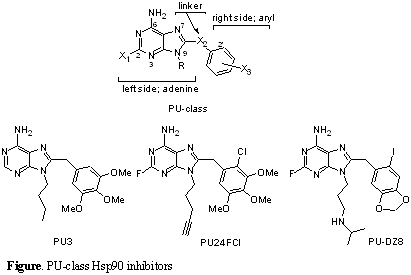
We have pioneered the development of synthetic Hsp90 inhibitors. Making use of the specific fold adopted by ATP upon binding to Hsp90, we were able to design the purine-scaffold class derivatives (PU-class, Figure) with Hsp90 inhibitory activities. The first synthesized derivative of this class, PU3, bound Hsp90 with moderate affinity and exhibited biological activities in the 50 uM concentration range (ChemBio 2001). Further efforts focused at improving the potency of this agent, have led to the synthesis of several compounds with improved activity in both biochemical and cellular assays (BioorgMedChem2002). One such compound, PU24FCl, is a selective inhibitor of tumor Hsp90 and exhibits anti-tumor activities in both in vitro and in vivo models of cancer (ChemBio2004). The biological effects of PU24FCl are demonstrated in the 2-6 uM concentration range. Recently we have disclosed the synthesis of several 8-arylsulfanyl, -sulfoxyl and -sulfonyl adenine derivatives of the PU-class (JMedChem2005). The study identified derivative PU-H71 as the most potent Hsp90 inhibitor of the purine-scaffold series published to date (EC50Hsp90 binding = 30 nM; IC50SKBr3 growth inhibition = 36 nM), and also as the compound of this class with highest selectivity for tumor vs normal cell Hsp90 (700 to 3000-fold). In addition, we have synthesized and evaluated a series of 8-benzo[1,3]dioxol-5-ylmethyl-2-fluoro-9-alkyl-adenine derivatives and identified derivative PU-DZ8 as another potent PU-class Hsp90 inhibitor (EC50Hsp90 binding = 50 nM; IC50SKBr3 growth inhibition = 57 nM). Both PU-H71 and PU-DZ8 are highly water soluble as salts (stocks of 10-50 mM in water can be made). Importantly, a significant correlation between Hsp90 binding and cellular activities has been recorded for these derivatives. These agents equipotently affect multiple tumor-specific aspects of oncogenesis regulated by the chaperone. In concordance with their higher affinity for tumor cells, in vivo, PUs accumulate in tumors while being rapidly cleared from normal tissue. A consistent relationship was observed between their tumor accumulation profiles and their effects on transformation-specific Hsp90 client proteins. Long-term administration of PUs to human tumor xenografted mice led to anti-tumor activity without toxicity to the host.