Imaging plays a central role in the management of biliary cancers and, while multidetector computed tomography remains the method of choice for imaging of most hepatobiliary tumors, researchers in radiology at Memorial Sloan Kettering continue to asses other methods in the quest to improve treatment options and success rates for these patients.
Multidetector computed tomography (CT) technology provides multiplanar reformatted images of exquisite spatial resolution, which are used to help delineate vascular involvement of hepatobiliary tumors for surgical planning and to define response during chemotherapy.
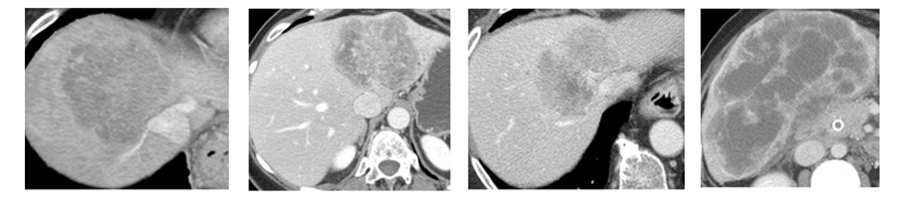
Imaging not only determines the location of a tumor - at the biliary confluence (Klatskin tumors), in the extrahepatic bile duct, or from the intrahepatic bile ducts – but can also help diagnostic radiologists classify biliary tumors as infiltrative, intraductal, or mass-forming, a morphologic distinction important to cholangiocarcinomas which informs surgical intervention. (1) Periductal infiltrative cholangiocarcinomas are subtle tumors that infiltrate along the bile ducts, whereas mass-forming intrahepatic cholangiocarcinomas (ICC) often present as large heterogeneous masses with capsular retraction and peripheral biliary dilatation (Figure 1). Intraductal cholangiocarcinomas are rarer still, but also have distinct appearances.
Advanced image processing with CT
The biological heterogeneity of ICC is evident both genomically and radiographically, but the question remains, are the two related to each other? In other words, do tumor genetics influence the appearance of ICC on medical imaging? To address this question, the HPB radiology team analyzed the heterogeneity of ICC on contrast-enhanced CT, using advanced image processing techniques. The field is often termed radiomics or texture analysis, and refers to the use of computer-based algorithms to measure differences in tumor appearance that are not quantifiable with the human eye. Our results show a spectrum of CT imaging patterns for ICC that correlate with markers of tumor hypoxia, as measured from pathologic samples (Figure 2). (3) Although preliminary, these encouraging results have driven us to investigate further relationships between imaging and genetics of ICC, in parallel with the exciting work performed by our collaborators in the Hepatopancreatobiliary Service at MSK.
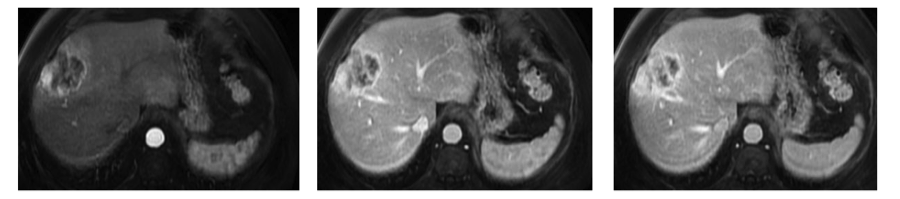
Advanced imaging with MRI
Magnetic resonance imaging (MRI) is often used as an alternative method when CT imaging is not possible (e.g., in a patient with iodinated contrast allergy), or when CT results are equivocal (e.g., in a patient who has possible intrahepatic metastases that would preclude surgical resection).
Added to that indication, MRI has potential for further use in ICC patients and that is to provide functional imaging techniques in the form of dynamic contrast-enhanced MRI (DCE-MRI) and diffusion-weighted imaging (DWI). Results from two previous clinical trials of hepatic arterial infusion (HAI) chemotherapy showed that with pre-treatment DCE-MRI measures of tumor perfusion we were able to identify patients with a longer survival following HAI chemotherapy. (2)
The MSK team is spearheading a reinvestigation of DCE-MRI in a new phase 2 clinical trial that combines HAI floxuridine (FUDR) with systemic chemotherapies (gemcitabine and oxaliplatin) for patients with ICC. The goal is to identify differences in tumor perfusion that can predict chemotherapy response, with the hypothesis that better perfused tumors receive greater amounts of chemotherapy.
DWI is another functional MRI technique that measures water diffusion (or motion) within organs or tumors. It has been used for decades in stroke imaging to identify areas at risk for brain ischemia. Over the last decade, it has been used in oncologic imaging as a research tool to predict tumor cellularity and response to treatment. Since the consequence of chemotherapy is often induction of cell death, the subsequent disruption of cell membranes removes the natural barriers to microscopic water motion. With DWI, we plan to measure treatment-related changes using apparent diffusion coefficients in ICC tumors, with the goal of guiding our management of ICC patients in real time.
