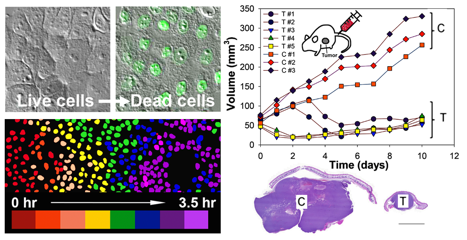
New research by investigators at Memorial Sloan Kettering and Cornell University suggests that very small silica nanoparticles are capable of killing cancer cells without the need for being loaded with toxic drugs. Under the right circumstances, the nanoparticles induce a form of programmed death in the cancer cells known as ferroptosis — a recently discovered biological process — that spreads from cell to cell in a wave-like manner.
The researchers showed that this approach caused cell death via ferroptosis in a number of cancer cell types and significantly shrank tumors and inhibited tumor growth in cancer-bearing mice. This lethal effect can be reversed by giving a drug that inhibits ferroptosis.
The study was led by MSK clinician-scientist Michelle Bradbury and cell biologist Michael Overholtzer and reported in Nature Nanotechnology, in collaboration with Cornell University and the University of Missouri. “This is the first time we have shown that the particle has intrinsic therapeutic properties without associated toxicity,” Dr. Bradbury says.
The nanoparticles, called C dots, are very small silica shells containing molecules of dye that glow brightly when hit by light of a specific wavelength. This allows doctors to trace their location as they move throughout the body. The nanoparticles can be tagged with molecules that bind to a receptor on the surface of cancer cells, lending them to powerful diagnostic and therapeutic applications.
Safe for Clinical Use
C dots are FDA-approved and have previously shown therapeutic promise in human patients with melanoma as well as animal models. They were developed by Ulrich Wiesner, a Cornell University professor, who, along with Dr. Bradbury, co-directs the newly established MSK-Cornell Center for Translation of Cancer Nanomedicine.
The discovery that C dots could naturally induce ferroptosis offers a potentially effective — and likely safer — strategy for treating cancer with nanoparticles. Conventional approaches have required the nanoparticles to be loaded with cancer-killing drugs on their surface.
The researchers are collaborating with clinicians to design new therapies using this approach in combination with standard therapies to treat cancer. “Cancers most sensitive to this mechanism are being evaluated so that we can target particles to them,” Dr. Overholtzer says.
“The hope is that treatment efficacy will be further enhanced by the addition of C′ dots before testing in humans,” Dr. Bradbury explains, adding “we are also harnessing the potential of these particles to regulate immune cells and the tumor microenvironment to stimulate anti-cancer activities.”