There is a real and significant need to improve clinical outcomes in patients with cholangiocarcinoma by building on first-line chemotherapy regimens and developing novel strategies for treatment in the second-line setting.
Cholangiocarcinomas are rare malignancies that arise from the epithelial cells of the bile ducts. They are highly lethal as they are often diagnosed at an advanced stage.
Further complicating the picture is that patients with advanced biliary tract cancer are a heterogeneous group, comprising both locally advanced and metastatic disease, as well as a variety of primary disease sites (intrahepatic bile ducts, extrahepatic bile ducts, gallbladder, and ampulla). This heterogeneity often complicates assessment of treatment efficacy.
Memorial Sloan Kettering is at the forefront of ongoing research and clinical initiatives to improve survival rates for patients with cholangiocarcinoma. For example we are one of only a few centers in the U.S to employ liver-directed therapies - using hepatic arterial infusion (HAI) - along with a number of systemic agents.
Systemic Combination Chemotherapy
MSK was involved in the seminal multicenter phase 3 ABC-02 trial (1) that randomized over 400 patients, with locally advanced or metastatic cholangiocarcinoma, gallbladder cancer, or ampullary cancer, to either combination therapy of cisplatin and gemcitabine or gemcitabine alone.
The median overall survival rate was almost 12 months with cisplatin and gemcitabine combined as compared to just over eight months with gemcitabine alone (p < 0.001). The median progression-free survival and response rates were also higher in the cisplatin plus gemcitabine group and the combination was well tolerated with an acceptable toxicity profile.
As a result, cisplatin plus gemcitabine has become a standard of care therapy in the first-line setting for patients with advanced biliary cancers. In practice, the cisplatin dose is frequently lowered to allow prolonged therapy, past six months, in patients with stable or responding disease who are tolerating treatment well.
Another combination therapy - gemcitabine with oxaliplatin - appears to have oncologic activity equivalent to the gemcitabine and cisplatin combination in advanced biliary cancer, and selection of one first-line regimen over the other is usually made based on toxicity profile.
Seeking Second-line Strategies
If a patient’s disease progresses on this first line treatment, second line treatment is less effective with response rates less than 10 percent. Agents targeting the epidermal growth factor receptor (EGFR) and vascular endothelial growth factor (VEGF) pathways, among others - have failed to show additional benefit in randomized trials. (2), (3)
Advancements in molecular profiling techniques at MSKCC (and elsewhere) using next generation sequencing have allowed identification of subsets of patients whose tumors harbor a potentially actionable genetic target, facilitating a more personalized strategy to determine suitability for a particular experimental approach. Distinct molecular alterations have been observed among intrahepatic and extrahepatic cholangiocarcinomas and gallbladder cancer, highlighting the molecular diversity within biliary cancers. Mutations in IDH1/2, chromosomal rearrangements involving FGFR2, ROS1, and NTRK, and mutations/amplifications in ERBB2 are examples of genetic alterations that could be used to guide assignment of a patient with biliary tract cancer to a particular clinical trial of targeted therapy
Optimally, all patients with biliary cancer considering treatment on a clinical trial would have molecular profiling of their tumor tissue to assess suitability for these ongoing studies. Currently, clinical trials are evaluating the use of inhibitors of IDH1/2, FGFR, and TRK, among others, in patients with biliary tract cancers harboring relevant genetic alterations.
To date, no study evaluating immunotherapy drugs such as pembrolizumab specifically for biliary tract tumors has been reported, but trials encompassing various solid tumors in patients with biliary carcinomas eligible for inclusion are ongoing.
The safety and antitumor activity of pembrolizumab in patients with programmed death-ligand 1 (1PD-L1)-positive biliary tract cancer was assessed as part of the ongoing multi-cohort, phase 1b trial using pembrolizumab monotherapy in patients with PD-L1-positive advanced solid tumors. (4) Of the 89 patients with biliary cancer screened for PD-L1 expression, 37 were considered to have PD-L1-positive tumors. Of those 37 patients, 24 were enrolled. Most patients had been exposed to gemcitabine plus cisplatin therapy prior to study enrollment. The overall response rate was 17.4 percent (95% CI, 5.0–38.8), including three patients with biliary tract cancer and one with gallbladder cancer. In patients with mismatch repair-deficiency, a response rate of 33 percent was seen. These results suggest that immunotherapy could have a role in the treatment of biliary tract and gallbladder cancers, at least for a subset of the patients, and additional studies evaluating immunotherapy approaches are ongoing.
Liver-directed Therapy
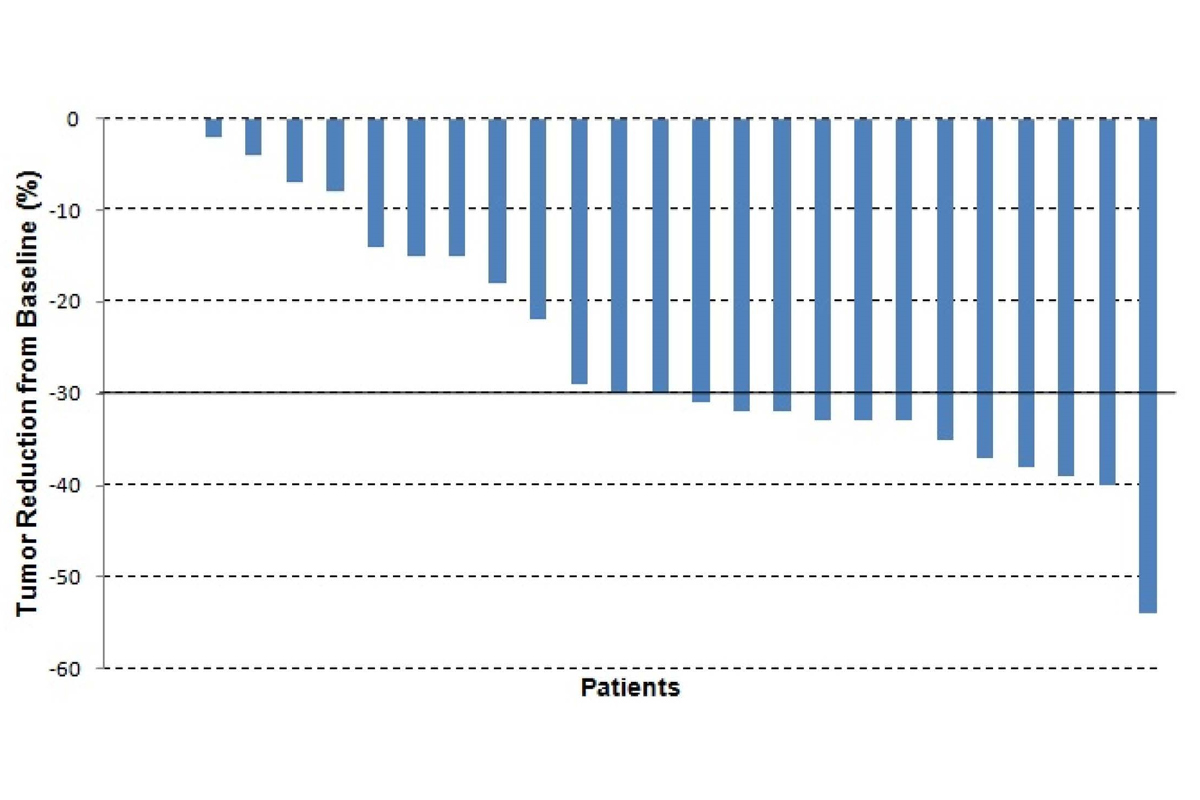
Figure 1. Maximum reduction from baseline using RECIST (tumor size change ≥ 30% considered a partial response) in patients who received HAI FUDR and dexamethasone.
Another avenue for treatment of intrahepatic cholangiocarcinoma is liver-directed therapy. We have conducted three studies at MSK evaluating the use of hepatic arterial infusion (HAI) chemotherapy to treat cholangiocarcinoma. In the first study, (5) floxuridine (FUDR) alone was infused over a two-week interval, followed by a two-week infusion of heparin/saline. This regimen showed a 53.8 percent response rate in 26 patients with intrahepatic cholangiocarcinoma (Figure 1). If patients with stable disease are included, then 96 percent of patients had either a response or stable disease. This group of patients had a median survival of 29.5 months. The addition of systemic bevacizumab to HAI FUDR did not improve the response rate but continued to demonstrate a good median survival of 31.1 months. (6) An ongoing study for patients with intrahepatic cholangiocarcinoma is evaluating the addition of a systemic chemotherapy, gemcitabine plus oxaliplatin, to HAI FUDR (Figure 2).
A large retrospective review of 525 patients with cholangiocarcinoma treated at MSK was reported in 2016 comparing the use of HAI therapy plus systemic therapy versus systemic alone. (7) Of patients with liver-only disease (n=104), 78 were treated with HAI and 26 were treated with systemic only. The median survival was 30.8 months for the HAI group and 18.4 months for the systemic group (p=0.001). The response rate was higher in the HAI group than in the systemic group, 59% and 39%, respectively (p=0.11). We are continuing to work with new agents and HAI therapy for cholangiocarcinoma.
Ongoing and future studies for treatment of cholangiocarcinoma will continue to make progress towards improved outcomes for these patients.
