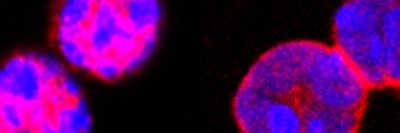
Cancer cells and immune cells have something very important in common: They both use a form of metabolism called aerobic glycolysis — also known as the Warburg effect — to acquire nutrients and energy.
This process breaks down glucose sugar into lactate in the cell’s watery cytoplasm without using oxygen or mitochondria, the cell’s so-called power plants. Scientists think cells resort to this relatively inefficient way of doing business for two main reasons: It provides a quick burst of usable energy and — perhaps even more important — gives the cells the building blocks needed to churn out more copies of themselves.
But a new paper published in the journal Science by researchers at the Sloan Kettering Institute (SKI) adds an intriguing new twist to what advantages the Warburg effect provides to immune cells — it helps to turn them on.
Nutrient Signals
Immune cells have a vital job to perform in the body. They must recognize dangerous invaders or cancer and then proliferate rapidly to respond to and contain the threat. The body’s main immune fighters are called T cells.
Since the late 1980s, immunologists have known that T cells require two signals to activate them — one through the T cell receptor itself, and one through a molecule called CD28. When both these wires are tripped, the cells begin a vigorous program of growth and differentiation, sucking up buckets of glucose in the process.
But as SKI immunologist Ming Li and his colleagues now show, they also dramatically increase their production of an enzyme that switches on aerobic glycolysis. The increased amount of this enzyme, called lactate dehydrogenase, shifts the cell’s metabolism toward the Warburg effect.
“Connecting co-stimulation to this metabolic reprogramming is a fundamentally new way to think about T cell regulation,” Dr. Li says. “It shows us how immune cells rewire their metabolic pathways to fit their appropriate function.”
Epigenetic Rewiring
The most surprising find was what effect this shift in metabolism has on gene expression — the turning on and off of genes. Dr. Li and colleagues, including SKI postdoctoral fellows Min Peng and Na Yin, discovered that the metabolites (breakdown products) of aerobic glycolysis participate in a process called histone acetylation, in which chemical tags called acetyl groups are attached to the proteins around which DNA is spooled in a chromosome. Acetylation promotes gene expression — in this case of a key immune gene called interferon gamma.
When the team knocked out the LDHA enzyme, preventing aerobic glycolysis from occurring, they found that the interferon gamma gene was turned off. And when they blocked the enzyme that controls histone acetylation, the gene could not be turned on.
Histone acetylation is what’s called an epigenetic modification — a change in how DNA is packaged in a chromosome that influences whether genes are expressed. Epigenetic mechanisms are increasingly recognized as an important link between the cell’s metabolism — its use of energy and nutrients — and its genetic program. A cell with abundant nutrients, for instance, may use epigenetic changes to turn on genes for cell division or some other resource-intensive task, thus ensuring that the cell’s genetic program matches its nutrient and energy capacity. But this is the first time that anyone has shown a link between this specific form of metabolism and this exact epigenetic mechanism.
Implications for Therapy
The fact that both cancer and immune cells share the same type of metabolism creates some tricky problems for those who would like to treat cancer by interrupting the way it obtains energy or nutrients.
“A lot of companies are developing inhibitors for LDHA, to try to starve cancer cells of nutrients and block tumor growth,” Dr. Li says. “But what our results suggest is that you can’t do this without also shutting off the antitumor immune response.”
Where Dr. Li thinks such inhibitors might be more useful is in treating autoimmune diseases, in which the immune system attacks normal tissues. His lab has already filed a provisional patent on this idea.
Dr. Li says the recent results underscore the need to think about cancer treatment in the context of other cells that might be affected. “I think people are starting to appreciate that when it comes to cancer treatment, we need to think more broadly, not just about tumor cells but about the tumor environment and the immune system as well.”