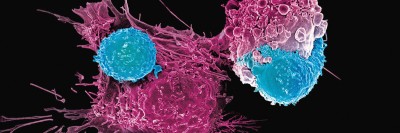
How MSK Is Teaching CAR T Cells to Attack Solid Tumors

When Marylou Barton heard her biopsy results, she held her daughter Kristin’s hand while they both sobbed in the doctor’s office. The mesothelioma — a rare and aggressive cancer that arose in the lining of Marylou’s lungs — appeared to have spread. She likely had about a year to live.
Marylou, then 71, had spent decades helping others in Pennsylvania — first as a child abuse prosecutor and then as an environmental lawyer. But it looked like nobody could save her.
“The physicians at my hospital said, ‘Just go home, get your will and all your papers in order,’ ” Marylou recalls. “It seemed like there was no hope.” It was February 2019.
Getting a Second Opinion at MSK for a Metastatic Mesothelioma Diagnosis
Her three daughters — all present when the biopsy was taken two days earlier — had other ideas. Kristin, Rosemary, and Sara searched the web for options and unanimously decided that Memorial Sloan Kettering Cancer Center (MSK) was the place to go for a second opinion. Soon, Marylou had an appointment with MSK’s multidisciplinary mesothelioma team, including surgeon Prasad Adusumilli, MD.
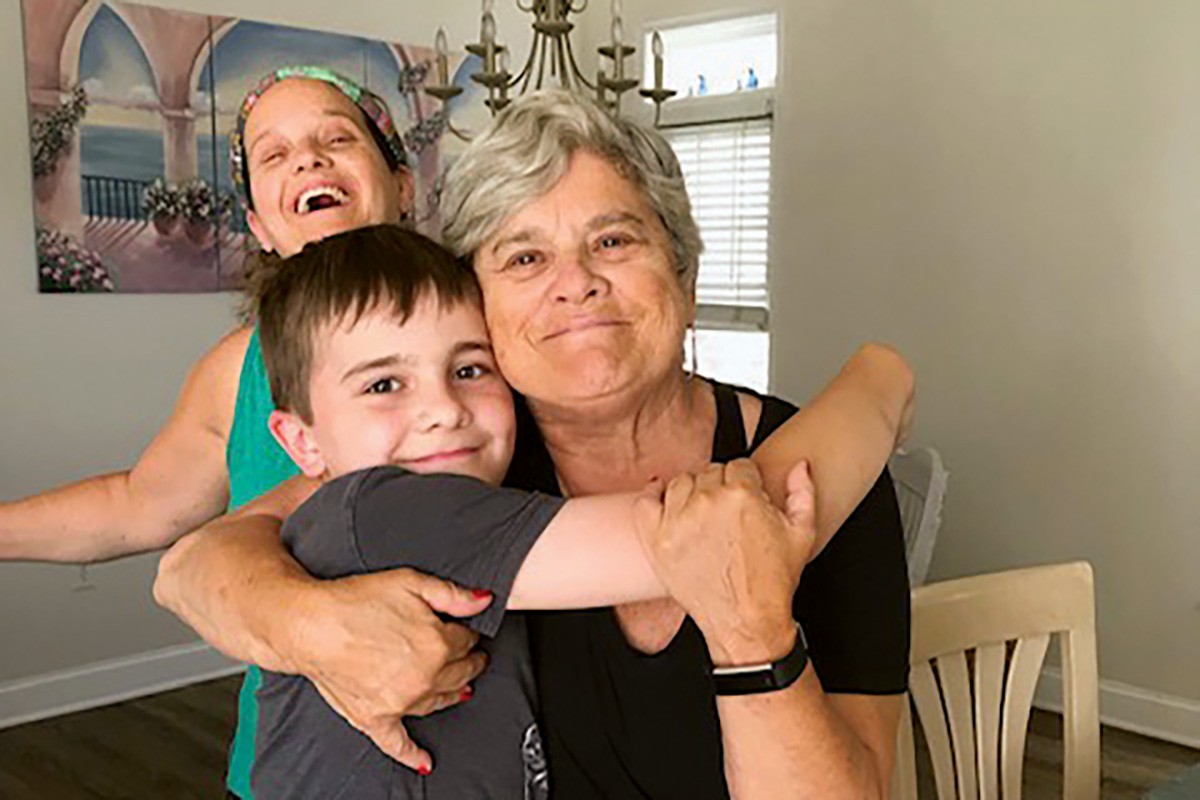
What they said to Marylou immediately gave her hope: Her cancer had not metastasized and could be held at bay with chemotherapy for the time being. Moreover, Dr. Adusumilli was gathering funds to start an innovative new mesothelioma clinical trial testing a new form of immunotherapy called chimeric antigen receptor (CAR) T cell therapy.
“I’m a positive thinker, and Dr. Adusumilli saw that in me — he said his grandmother had always told him that was half the battle,” Marylou says. “He said, ‘When we get the money for this are you interested?’ And I said, ‘Definitely.’ ”
What Is CAR T Cell Therapy, and How Does It Work?
CAR T cell therapy involves removing immune T cells from a patient and outfitting them in the lab with receptors that recognize specific targets — known as antigens — on the surface of a cancer cell. It has shown great promise for the treatment of blood cancers such as leukemia and lymphoma. But it had been much less effective against diseases such as mesothelioma and other solid tumors, which account for most cancers.
Getting Funding for Solid Tumor CAR T Cell Therapy Clinical Trial
Dr. Adusumilli was determined to tackle the solid tumor challenge. Thanks to funding from the Baker Street Foundation, he launched the mesothelioma clinical trial later that year. Marylou finished her chemotherapy and received her first CAR T cell treatment in the fall.
After receiving the CAR T cells, she was able to complete the last phase of her mesothelioma treatment back home at a hospital in Lancaster, Pennsylvania, where she received an immunotherapy checkpoint inhibitor drug called pembrolizumab (Keytruda®). Today, nearly five years after her treatment, Marylou is still going strong. “I would not be alive today if I did not come to MSK,” she says.
Why Solid Tumors Are Hard for CAR T Cells to Treat
Marylou’s mesothelioma treatment was one of the first in an exciting new wave of CAR T therapies for solid tumors. These cancers have been largely impervious to CAR T cell therapy because they are often difficult for T cells to penetrate. They also have a surrounding ecosystem — called the tumor microenvironment — that blocks CAR T cells from attacking until they just run out of steam, a state known as T cell exhaustion.
Tumor Microenvironments and CAR T Cell Exhaustion
This resistance has frustrated clinicians, who see that the CAR T cells detect the tumor but then appear to weaken and fail to finish the job.
“In order for CAR T cell therapy to work against these tumors, we need the T cells to be effective at infiltrating the tumors, killing enough cancer cells, and staying active long enough to stop the cancer from coming back,” Dr. Adusumilli says.
Injecting Car T Cells Directly at Tumor Site
Over the past decade, Dr. Adusumilli has made several important advances to overcome these hurdles. One solution, when possible, is to deliver the CAR T cells directly to the tumor site rather than infusing them into the bloodstream. Dr. Adusumilli’s intervention radiology colleagues developed several new techniques to inject tumors without any complications.
To treat mesothelioma with CAR T Cells, that means injecting the cells directly into the pleural cavity — the area outside the lungs and along the pleural lining, which is a membrane that covers the inside of the chest cavity and surrounds the lungs. That’s how Marylou received her cells.
“They rolled me on my side and injected it into my back, to an exact spot where the cancer was next to my lung,” she says. “It was just one procedure, and I didn’t feel a thing.”
3 Future CAR T Cell Innovations Being Explored in the Lab
Dr. Adusumilli’s laboratory has explored additional ways to make the T cells themselves more effective.
1) CAR T Cells Plus Radiation: This approach uses low-dose radiation on the tumors to help the CAR T cells penetrate them.
2) Adding Decoy Receptors: Another trick is genetically engineering the CAR T cells to contain a “decoy receptor” that stops cancer cells from blocking the immune cells before they become exhausted.
3) Adding c-KIT Mutations: Perhaps the most audacious strategy involves bolstering the strength of the CAR T cells by stealing one of cancer’s own weapons: a genetic mutation in a gene called c-KIT that is critical to the ability of cancer cells to grow, multiply, and survive. In lab studies, adding mutated c-KIT to the CAR T cells enabled them to gain strength and fight the tumor longer. The researchers could regulate the c-KIT effect with a drug, like turning a volume knob up or down. This should allow the therapy’s power to be harnessed more safely.
Dr. Adusumilli’s team appreciates the irony of turning cancer’s weapons against it. A research brief written by Dr. Adusumilli and MSK colleague (and study co-author) Meriem Taleb, PhD, in Nature Cancer concludes with a quote from the Dalai Lama: “Your enemy is your greatest teacher.”
A Prestigious Grant Funds MSK’s CAR T Cell Research for Solid Tumors
These latest innovations tested in the lab are now getting support for testing in patients. In March 2024, the National Cancer Institute recognized Dr. Adusumilli’s pioneering work by awarding him a prestigious grant to pursue these new approaches in a clinical trial.

The substantial five-year award will support his efforts to incorporate all three lab innovations into a multicenter study treating patients with pleural cancers — those that affect the area outside the lungs in the chest cavity. If successful, the trial could represent a major milestone for treating many malignancies, in addition to mesothelioma.
In receiving the grant, Dr. Adusumilli credits his collaboration with MSK colleagues, including physician-scientist Michel Sadelain, MD, PhD, a pioneer in CAR T research, as well as medical oncologist Michael Offin, MD, and radiation oncologist Daniel Gomez, MD.
“Although we are starting with pleural cancers, we could potentially expand this to most people with advanced solid tumors,” Dr. Adusumilli says, “especially those resistant to other treatments such as chemotherapy and immunotherapy.”
Marylou Is Thriving After Surviving Mesothelioma
As for Marylou, she’s now retired and continues to make the most of her second chance, spending time with her daughters, five grandchildren, and many friends. She also loves to travel.
“I went to Sicily last fall for three weeks, Jamaica in February for five days, and I’m about to go to Florence soon,” she says.
Both she and Dr. Adusumilli are very grateful for the philanthropic support that brought the research this far — and will continue to fund further research — especially from the Baker Street Foundation.
“Philanthropy is critical for this work because it supports high-risk, high-reward strategies that might not otherwise be funded by the traditional funding sources,” Dr. Adusumilli says.
Marylou says she is forever grateful to be a beneficiary of someone else’s generosity and altruism after a life of serving others.
“It’s kind of like ‘what goes around comes around,’ in a positive way. And here I am, on the receiving end of it, enjoying every moment of my life.”
Dr. Sadelain holds the Stephen and Barbara Friedman Chair.
Dr. Adusumilli holds the Min H. & Yu-Fan C. Kao Chair in Thoracic Cancer