Molecular biologist Agnel Sfeir studies how DNA is broken and repaired, and how these processes are related to aging, cancer, and other phenomena. A recent interest of hers is in the biology of the DNA inside of mitochondria — the “power plants” of the cell. She calls this DNA “the forgotten yet fascinating genome.”
Dr. Sfeir joined Sloan Kettering Institute’s Molecular Biology Program in March 2021, having previously been a faculty member at New York University’s Skirball Institute of Biomolecular Medicine. In an interview, she spoke about her research interests and what motivates her as a scientist.
When did you know you wanted to be a scientist?
In high school, I was really good at math but I enjoyed biology a lot. I was torn between becoming an architect or studying life sciences — even though the two disciplines could not be more different. Ultimately, I settled on biology and did my undergrad at the American University of Beirut.
I grew up in Lebanon, where science is nowhere close to the caliber of science in the States. Back then, there were no PhD programs, and getting a bachelor’s degree in biology was seen as sort of a dead-end career move. To convince my parents, I feigned an interest in applying to medical school afterward, but my real goal was always to move to the US for graduate school. I ended up moving to Texas in 2001 and joined the PhD program at University of Texas Southwestern.
What is the focus of your lab’s research?
We’re interested in how cells repair damaged DNA. To me, DNA is the most important molecule of life. But there is a paradox about DNA: As a molecule it’s very unstable, yet the genome as a whole is very stable. The reason for this is that our cells dedicate several repair pathways to fix DNA when it breaks. But these repair pathways, which operate almost flawlessly in a normal cell, are often mutated and disrupted in cancer.
In the lab, we want to understand how these repair pathways operate in a normal cell and how they go wrong in cancer. Can we take advantage of this vulnerability and use the fact that DNA repair has gone wrong in cancers to develop better therapies?
I was trained as a telomere biologist. Telomeres are the structures at the ends of chromosomes. When I started my lab at the Skirball Institute in 2012, I felt I needed to think of something new and different. I took two months off from reading my usual scientific literature and started digging into new literature. I always had a curiosity about mitochondrial DNA. As I read more, it become evident that there are a number of fundamental questions related to mitochondrial DNA stability and maintenance that are unanswered. So, I decided to venture out of the nucleus. Currently, a third of my lab works on the stability of mitochondrial DNA, a third works on DNA repair, and a third works on telomeres.
What do you want potential trainees to know about your lab?
I like to be challenged as a mentor, and I push my team to challenge my ideas and interpretations. What I like most about my group is that they have tremendous team spirit and they are super fun to work with. We have a chess set in the lab that we are bringing with us to SKI and we need to find room for it in our new space on the 11th floor of the Rockefeller Research Lab building. I admit, although grudgingly, that I am the worst chess player in the lab.
Jokes aside, to my students and postdocs, I’m a mentor for life. People who leave my lab always remain part of the family. It is very important for me that everyone who leaves my lab is successful at whatever path they take, whether that’s academia or industry.
You recently published a paper on mitochondrial DNA. What did you find?
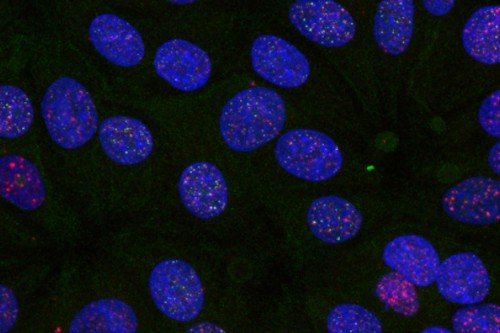
The question we asked is the following: Does the nucleus take note when the mitochondrial DNA is damaged? To address this simple but fundamental question, we looked at what happens to nuclear gene expression when we induce breaks in mitochondrial DNA. Our data showed strong activation of innate immunity genes. It’s as if the mitochondrion all of a sudden is perceived as a foreign entity in our cell.
Then we asked: How is this response being triggered? It turns out that following breaks to mitochondrial DNA, disruptions of the mitochondrial membrane allow the release of mitochondria DNA and RNA to the cytoplasm. While mitochondrial DNA is degraded, mitochondrial RNA that accumulates in the cytoplasm activates RNA-sensing machinery and triggers an immune response.
Does this discovery have implications for cancer treatment?
That’s when we had an “aha!” moment. Because when tumor cells are treated with DNA-damaging agents to kill cancer — for example, radiation therapy — part of the therapeutic response to these treatments comes from the triggered immune reaction. Following irradiation, innate immunity is activated and prompts T cells to clear the tumor.
In the past decade or so, it has been assumed that this innate immune response is inherent to breaks to the nuclear DNA. But we did a simple experiment. We took cells in culture and depleted them of mitochondrial DNA and then irradiated them. To our surprise, there was no innate immune response at all, as if the mitochondrial DNA is actually what is priming the immune response.
We then did a more refined experiment where we introduced breaks in the mitochondria DNA alone, the nuclear DNA alone, or both together. And what we noticed is if you break nuclear DNA alone, activation of innate immunity is minimal. Breaks to mitochondria DNA activate somewhat, but when combined they synergize.
So, the conclusion is that the mitochondrial DNA breaks prime an immune response in the cells. This forgotten genome, which no one seemed to worry about in terms of the cellular response genome instability, is actually critical when you’re treating cancer cells with radiation and perhaps other genotoxic agents.
Do you have a lab motto or philosophy?
To start off: Identify a simple but important question. Second: Develop tools, methods, models, or whatever innovative technology it takes to answer this simple question. Finally: Be fearless and never give up until you nail it.