I am a physician-scientist who focuses on the development of new multi-modal imaging approaches for specific applications, such as sequential in vivo imaging studies in cancer biology, cancer immunotherapy, and radiation sciences.
My research aims to develop (a) new approaches for repetitive in vivo optical, nuclear, and MR imaging of the fate of cancer-specific immune cells after their adoptive transfer for antitumor immunotherapy (done initially in animals and then in patients); (b) novel assessments of targeted drug therapies based on imaging of specific signal transduction pathways pre- and post-treatment, with the goal of rapid translation into relevant clinical studies; (c) new multi-modal genetic reporter systems for different imaging technologies to be combined during the course of the same study and in a way that harnesses the best features and utilities of each of the modality.
Imaging of Genetically Modified Tumor-Specific T Cells in Prostate and Colon Cancers
The overall aim is to develop approaches for repetitive in vivo imaging of the fate of T lymphocytes after their adoptive transfer for antitumor vaccination. It is obvious that continuous whole body monitoring of the efficacy of many adoptive cell-based therapies can not be done by invasive methods (e.g., multiple biopsies) except for blood sampling.
In our opinion, noninvasive whole body imaging would significantly aid in the development and clinical implementation of cell-based therapeutic approaches by providing the means for noninvasive monitoring of the fate of the injected T cells over a long period of observation. Imaging could help to address several questions related to T cell migration and homing to the tumor target, their long-term viability, and their subsequent activation and cytolytic activity.
The ultimate aim of noninvasive imaging of the degree of T cell activation at tumor target is to assess cytolitic potential of tumor infiltrating T cells and predict the tumorolytic efficacy early on during therapy in clinical setting.
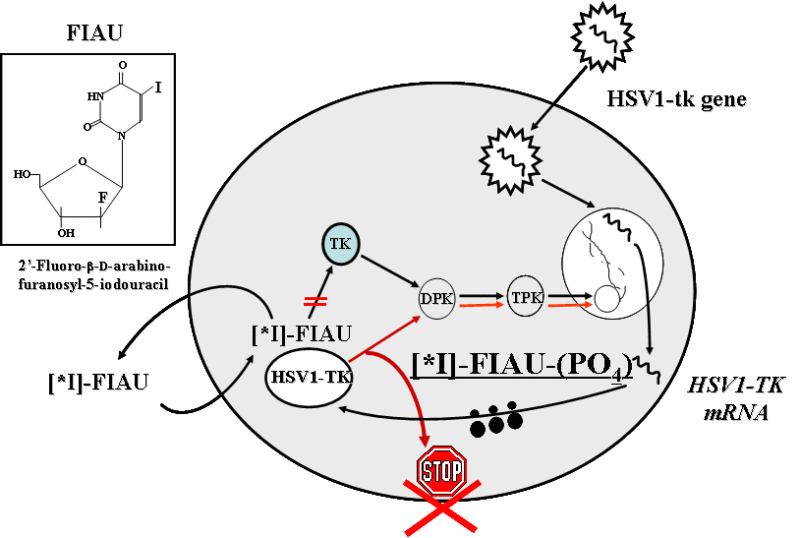
The paradigm of noninvasive reporter gene imaging involves the administration of a radiolabeled probe that is selectively bound or metabolized (e.g., phosphorylated) and trapped by interaction with the gene product (e.g., an enzyme) in the reporter gene-transduced cell. In this paradigm, the level of probe accumulation is proportional to the level of reporter gene product expressed.
We recently demonstrated the feasibility of serial PET imaging of antigen-specific T cells bearing an HSV1-tk reporter/suicide gene. In this work, we explore the feasibility of genetic labeling of T lymphocytes with novel reporter genes/probe combinations and novel multi-reporter gene constructs that would allow for repetitive noninvasive in vivo PET imaging of T cell trafficking and activation. We have developed several dual-reporter systems in which 1 reporter gene will be expressed constitutively as a “beacon” gene for imaging of the localization of adoptively transferred T cells; while the second reporter will be expressed as a “sensor” gene in a T cell- and activation-specific manner, thus allowing for visualization of activation and functional properties of T cells.
The proposed multi-reporter gene imaging paradigm will be implemented in the assessment and optimization of new anticancer T cell vaccines that utilize anti-CEA or anti-PSMA artificial T cell receptors in experimental therapies of colorectal and prostate carcinomas, respectively. Imaging will be used to monitor trafficking, localization, and activation of adoptively transferred T cells targeted against colon and prostate carcinomas before and during the co-stimulatory therapy with various cytokines.
Human Reporter Genes
![In vivo PET imaging of hƒ´TK2 expression in transduced and wild-type U87 xenografts in mice at 2 hours after [124I]FIAU administration.](/sites/default/files/node/11622/images/figure-2.jpg)
An important requirement for a long-term genetic labeling of immune stem/progenitor cells in humans is that the reporter gene product should not be immunogenic in order to avoid the possibility of rejection of transduced cells. In preparation for potential clinical applications of reporter gene imaging, we have developed (and are in the process of developing) novel nonimmunogenic reporter genes that are of human origin for long-term repetitive imaging in patients. One of them is human truncated mitochondrial thymidine kinase type 2 (hDTK2), which is likely to be nonimmunogenic in humans; and could be readily translated into a clinical setting for PET imaging with [124I]FIAU or other novel nontoxic highly specific probes, such as 18F- or 11C-radiolabeled FEAU. hDTK2 may also be used as a suicide gene due to its ability to phosphorylate clinically used nucleotide analogs.
Multimodality Imaging Approaches
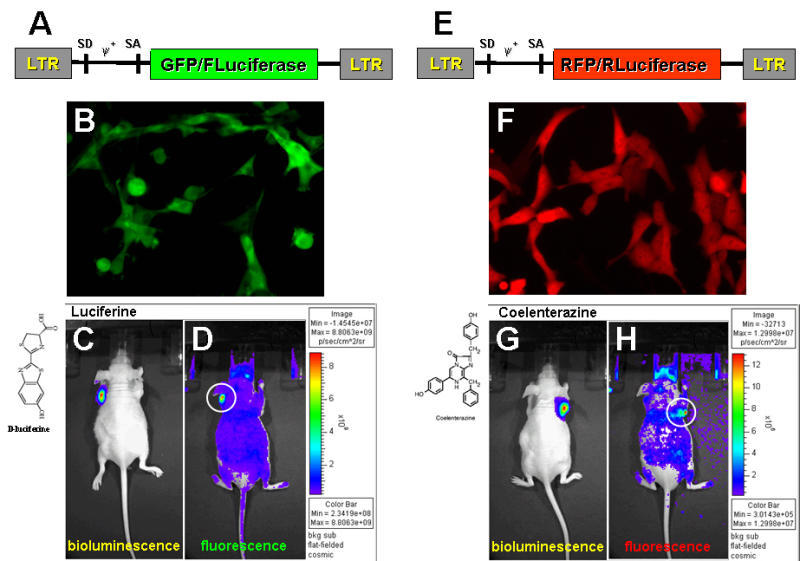
Multimodality imaging approaches allow for different imaging technologies to be combined during the course of the same study and harness the best features and utilities of each of the modality. The advantages of hybrid reporter genes that allow for multimodality imaging in vivo have been recognized as well. Initially, the advantages of multimodality imaging using herpes simplex virus type one thymidine kinase (HSV1-tk) for nuclear imaging and green fluorescent protein (GFP) for optical fluorescent imaging were demonstrated. It allows for optical microscopic and whole body fluorescence imaging, as well as PET imaging. The fusion of bioluminescence and fluorescence reporter genes has also been reported.
In a recent publication, we described the HSV1-tk/GFP/Firefly luciferase (TGL) triple reporter construct that preserves the functional activity of its subunits and allows for seamless transition from fluorescence microscopy and FACS to whole body bioluminescence imaging, nuclear (PET, SPECT, gamma camera) imaging, and back to in situ fluorescence image analysis. Currently we are developing several fusion reporter genes bearing functional components for fluorescent and bioluminescent imaging in the same animal. When validated, these approaches will have direct applications to various imaging studies where 2 molecular events need to be tracked simultaneously, including cell trafficking of 2 or more distinct cell populations, gene therapy vectors, and indirect monitoring of several endogenous genes through the use of these reporter genes. These fusion reporter genes will allow for a seamless transition from one imaging modality to the other and facilitate cross-interpretation of results obtained with various in vitro, in situ, and in vivo imaging modalities.