Even as experts debate who deserves credit for developing CRISPR, progress using the powerful genome-editing technique is speeding ahead.
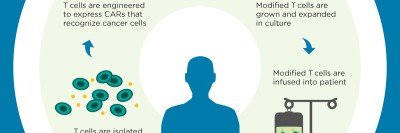
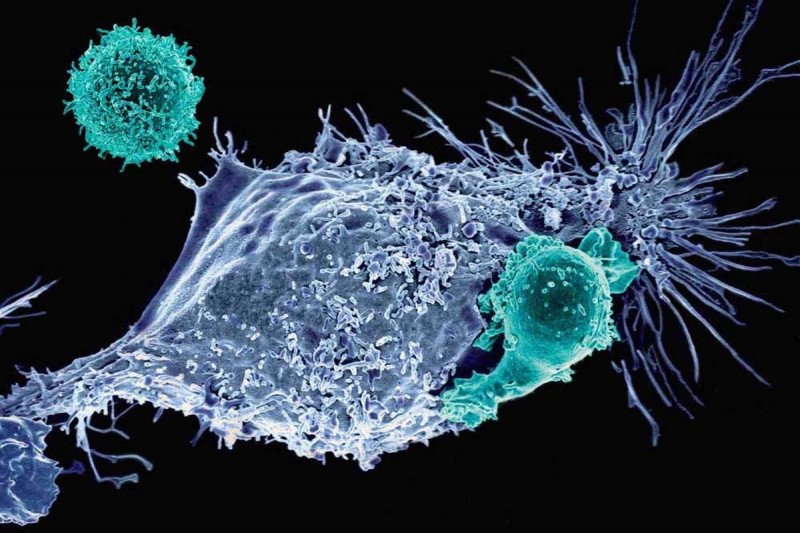
The latest advance involves using the technology to build chimeric antigen receptor (CAR) T cells, a type of immunotherapy for cancer. In a new study published today in the journal Nature, Michel Sadelain and colleagues show how CRISPR can be used to create CAR T cells with improved performance — packing more punch against tumors in mice.
“These CRISPR-engineered CAR T cells seem to have an optimal level of functioning,” says Dr. Sadelain, who directs the Center for Cell Engineering at Memorial Sloan Kettering. “They retain their ability to kill tumor cells for much longer than conventional CAR T cells, which tend to burn out more quickly.”
CAR T cells have garnered acclaim over the past few years thanks to their stunning success in treating several types of advanced blood cancers, including acute lymphocytic leukemia and chronic lymphocytic leukemia. The approach, which was pioneered at MSK, involves equipping a person’s own T cells with special receptors that can find cancer in the body and initiate an immune reaction against it.
To date, most CAR T cells are made using a retroviral technology that delivers the CAR gene to the immune cells. This delivery method results in the CAR gene being inserted at random into the genome of the recipient T cells. Because there can be unwanted genetic side effects that result from this somewhat scattershot approach, researchers are interested in developing more-precise delivery methods — FedEx for DNA.
In their new paper, Dr. Sadelain and colleagues — including two postdoctoral fellows from his lab, Justin Eyquem and Jorge Mansilla-Soto — show that they can use a popular version of the CRISPR technology called CRISPR/Cas9 to put the CAR gene right where they want it, producing cellular cancer fighters with improved killing power.
Location, Location, Location
The team initially tested a few different genome addresses before deciding upon a particular region called TRAC, which stands for “T cell receptor alpha constant.” This region contains the gene for a part of the immune cell’s main detector of foreign proteins: the T cell receptor for antigen. Using CRISPR, the team was able to slice open the DNA at this location then slip in their new gene — the one for the CAR.
CAR T cells made in this fashion have some remarkable properties. Not least, they are more effective at killing human tumor cells in a mouse model of cancer. Dr. Sadelain’s team found that the improved killing could be traced to the fact that the cells are less likely to become exhausted and so retain the ability to keep on fighting for longer. (“Exhaustion” is a term immunologists use to describe T cells that express molecules that tamp down their activity. PD-1, a common target of other immunotherapies, is one such molecule.)
“The TRAC locus works really well as a genome address,” Dr. Sadelain says. “You get the most out of your CAR when you express it from this location.”
Taming the Beast
The cells’ improved performance reflects the placement of the CAR under control of the regulatory machinery that normally governs the immune response to pathogens and cancer. Because of this precise positioning, the cells can turn the CAR on and off in a more natural fashion. In conventional CAR T cells, the CAR is on all the time, which can cause the cells to start out strong but then quickly lose steam.
“In a way, they’re tamer cells,” Dr. Sadelain says. “They don’t go wild and that’s why they last longer.” And, because they last longer, you ultimately need fewer of them, which should make manufacturing easier, he says.
A second promising attribute of these cells is the result of what Dr. Sadelain calls the “two-in-one strategy”: He and his colleagues used CRISPR to both add the CAR to the TRAC locus and, at the same time, interrupt the T cell receptor gene, making assembly of a functional T cell receptor impossible. Knocking out this receptor means that it may be feasible to make CAR T cells using cells from a genetically unrelated donor, without worrying about a serious immune complication called graft-versus-host disease — when the donor immune cells attack the recipient’s normal tissues as foreign.
Even off-the-shelf CAR T cells that could in principle work for anyone are a possibility with this approach, as discussed by Marcela Maus, Director of Cellular Immunotherapy at Massachusetts General Hospital, in an accompanying editorial in Nature.
Leading the Pack
Though these results are certainly exciting, Dr. Sadelain says the ultimate test of these CRISPR’d cells will be when they are infused into human patients. The next step in this line of research will be to conduct a clinical trial to compare the safety and efficacy of CRISPR-built cells with conventional CAR T models. Two such trials, for people with B cell malignancies, are currently being planned at MSK.
The use of CRISPR-modified cells in people would represent a true milestone in biotechnology, one that could serve to prod the entire field of genetic engineering forward. “The CAR field is likely to serve as a major testing ground for this emerging genome-editing technology,” Dr. Sadelain says.