For the second time in recent months, the US Food and Drug Administration has green-lighted a novel form of immunotherapy, called CAR T cell therapy, for the treatment of cancer. The approval is for axicabtagene ciloleucel (trade name Yescarta) for the treatment of people with aggressive diffuse large B cell lymphoma (DLBCL), a type of non-Hodgkin lymphoma. The cell-based product is made by Kite Pharma, which was recently acquired by Gilead Sciences.
A similar product from the drug company Novartis was approved in August for pediatric leukemia. Both cell-based treatments use genetically engineered versions of a person’s own immune cells to fight cancer. MSK researchers played a leading role in developing the technique.
DLBCL is the most common type of non-Hodgkin lymphoma, a blood cancer that primarily affects older adults. The typical treatment is chemotherapy using a combination of different drugs. This treatment may provide a cure but not always.
Previously, when a person relapsed after having therapy or stopped responding to treatment, a stem cell transplant was the only option. Now, CAR T cell therapy is a second potentially life-saving choice for people who may not be eligible for a stem cell transplant, or even for those whose disease has relapsed after having had one.
“For more than three decades, there has not been a single drug approved by the FDA for people with relapsed DLBCL,” says Anas Younes, Chief of the Lymphoma Service at MSK. “The approval of axicabtagene is a major step forward, providing a new effective treatment option for these patients. Our group is at the forefront of developing second-generation CAR T cells to further improve their efficacy.”
The FDA based its approval of axicabtagene ciloleucel on a clinical trial, led by Kite. The trial found that nearly half of people with this aggressive type of chemotherapy-resistant DLBCL had a complete response after receiving one infusion of CAR T cells, meaning all signs of their disease disappeared (at least for a time).
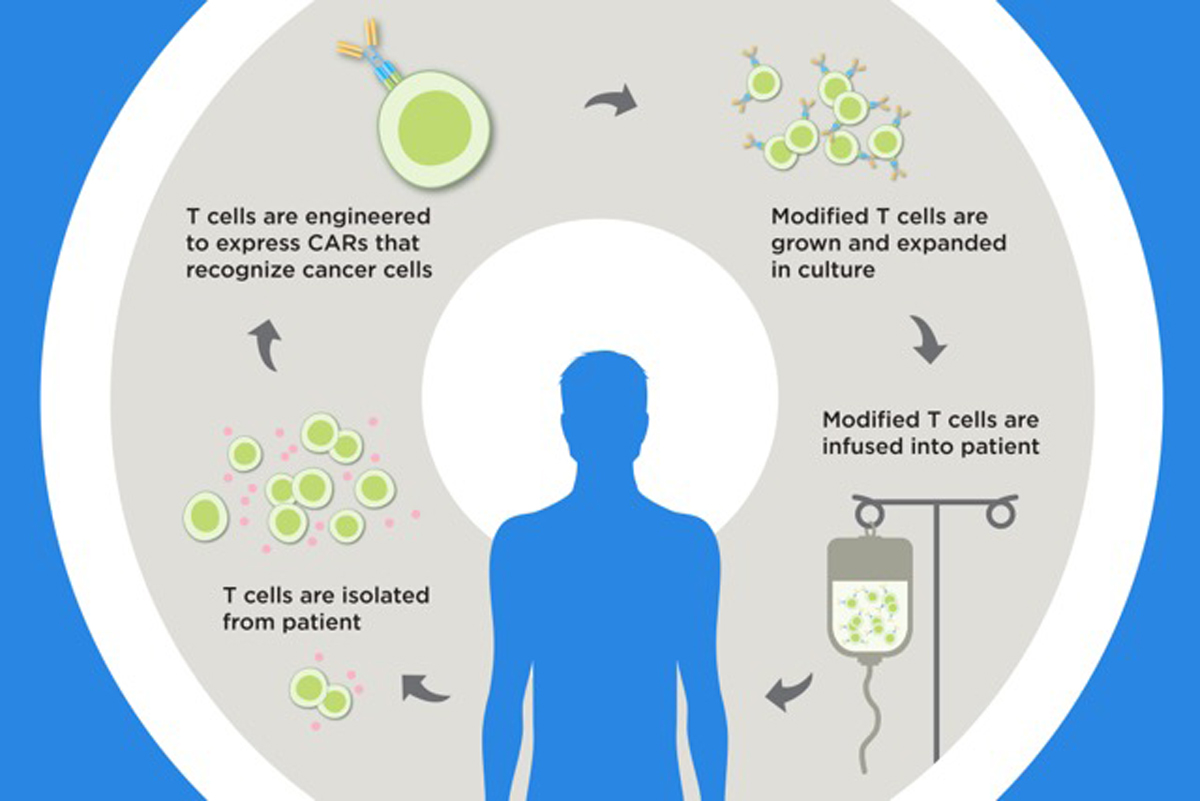
To make the cell product, doctors collect T cells from a person’s blood. The cells are frozen and then shipped to a lab where they are genetically engineered to contain a new gene. The modified cells are grown in the lab until there are billions of copies and then shipped back to the hospital for reinfusion into the person’s blood through an IV days later.
MSK is one of only a handful of cancer centers that have the experience and expertise necessary to administer CAR T cell therapies safely to patients.
“We have a multidisciplinary team of experts with vast experience who consult on every case,” says Sergio Giralt, Chief of the Adult Bone Marrow Transplant Service at MSK. “Our primary job is making sure that each patient gets the very best care — whether that’s CAR T cell therapy or another approach.”