For people with a common cancer-associated mutation called BRAFV600E, targeted therapies that block the effects of this genetic abnormality can be remarkably effective. Tumors shrink rapidly and dramatically. Unfortunately, most people eventually stop responding. Their tumors develop resistance to the medications and begin to grow again without restraint.
The central problem is one of tumor evolution. Like every form of life, cancer cells evolve in response to selective pressures, leading to genetic changes in the population. What Charles Darwin found to be true of finches, barnacles, and bonobos applies equally well to cancer cells. Mutations are continually arising in a population of cells. When a mutation helps a cell’s growth and reproduction, it becomes more common in the population. This is how drug resistance can emerge.
Drug resistance isn’t a problem only in cancer. Infectious diseases, caused by microbes, also develop resistance to commonly used drugs. But lessons learned in the battle against bugs are helping cancer doctors devise strategies for combatting resistance.
“The success of combination antiretroviral therapy in combating HIV infection, as well as other progress made in combating antibiotic resistance, has strongly influenced our work,” says Piro Lito, a physician-scientist at Memorial Sloan Kettering and the senior author of a new study published in Nature Medicine. “These examples show that understanding the evolutionary basis of resistance is key to a successful treatment strategy.”
To study the evolution of resistance, Dr. Lito and his colleagues transplanted human lung cancer and melanoma tumors with BRAF mutations into mice, making what are called xenografts. They found that a combination of three targeted drugs was effective at preventing the emergence of resistance in cancer cells. These results have immediate implications for the design of cancer drug regimens.
Route to Resistance
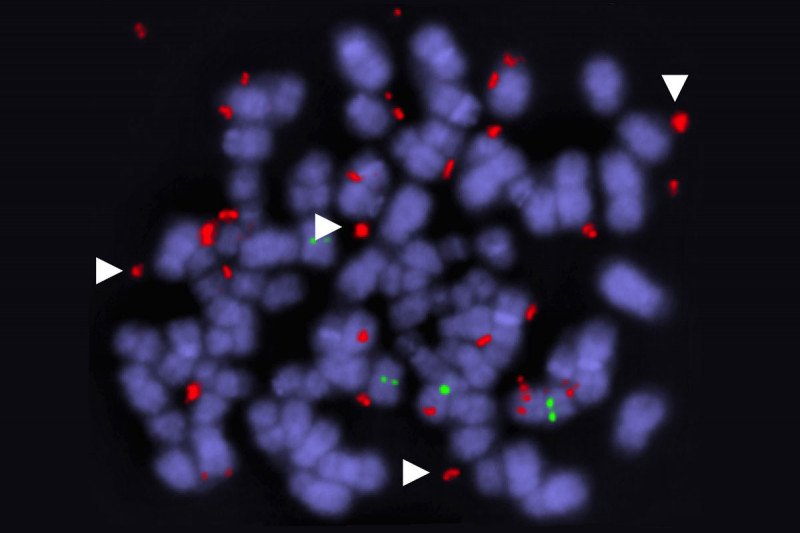
While the fact of drug resistance has been amply documented, less is known about how exactly it unfolds. Using their xenograft models, the MSK researchers were able to document a specific method by which the cancer cells outsmart the targeted drugs: They produce duplicate versions of the mutated BRAF gene. Cells with several BRAF copies have a competitive advantage over cells with only one. In essence, cancer cells get around the targeted drugs by making more of the BRAF protein that drives their growth.
These duplicate genes are found on circular fragments of DNA that are separate from the cells’ normal set of chromosomes. Cancer cells often contain these “extrachromosomal” genes, explains Jenny Xue, a Weill Cornell/Rockefeller/MSK Tri-Institutional MD/PhD student in the Lito lab and the paper’s first author. Typically their main purpose, she says, is to carry an oncogene that drives tumor growth.
The team used a powerful technique called single-cell DNA sequencing to peer into individual cells. They were then able to map the cells’ evolution in real time. An unexpected finding was the discovery that these BRAF amplifications occurred and were selected in multiple lineages of cells at the same time. This allowed tumors to maintain their genetic heterogeneity — and thus, their capacity for future evolution — while also permitting them to adapt to their immediate environment.
Raising a Barrier
For people with BRAFV600E-mutant cancers, including melanoma and lung cancer, the current standard of care is a combination of two drugs that target different parts of the BRAF signaling pathway. The two drugs are a RAF inhibitor and a MEK inhibitor. A third type of targeted drug, called an ERK inhibitor, is entering clinical testing. ERK inhibitors could in principle be used after people develop resistance to the other two types of drugs, which is quite common.
Giving targeted drugs sequentially may be effective in some instances, but the new research suggests that this is not the ideal approach. This stepwise administration provides an opportunity for the cells to evolve resistance, ultimately short-circuiting the new drug’s effectiveness.
“Our work and that of other investigators suggests that sequential therapy with drugs targeting the same pathway is not optimal,” Dr. Lito says. “We believe that the ideal approach to stopping tumor growth is to maximally inhibit the growth-promoting pathway that is driven by the mutant BRAF protein by attacking it from multiple sides at once.”
That means using all three drugs in combination at the same time, each targeting a different part of the BRAF pathway. This one-two-three punch makes it very difficult for tumors to grow. While a tumor cell might acquire a mutation that allows it to outsmart one of the drugs, the odds of it being able to outsmart all three drugs at the same time is small.
But what about side effects? Any time you combine medications, you raise the prospect of additional toxicity. To lessen this possibility, the researchers explored giving the three-drug combo with breaks between the cycles. They found that spacing apart the drug cycles in this manner was easier on the mice and also did not impair the drugs’ effectiveness. In fact, the spacing helped to reduce the ability of the tumors to adapt to the treatment, further decreasing the chance that resistance would evolve.
Dr. Lito says that he and his MSK colleagues are currently planning a clinical trial that will test the intermittent three-drug administration strategy in people.