
Men having prostate cancer surgery often fear side effects that can result from the procedure. Removing the tumor risks damage to surrounding nerves, resulting in erectile dysfunction and incontinence. Now a new way to light up nerves during the operation may help surgeons avoid this nerve damage, preserving the patient’s quality of life.
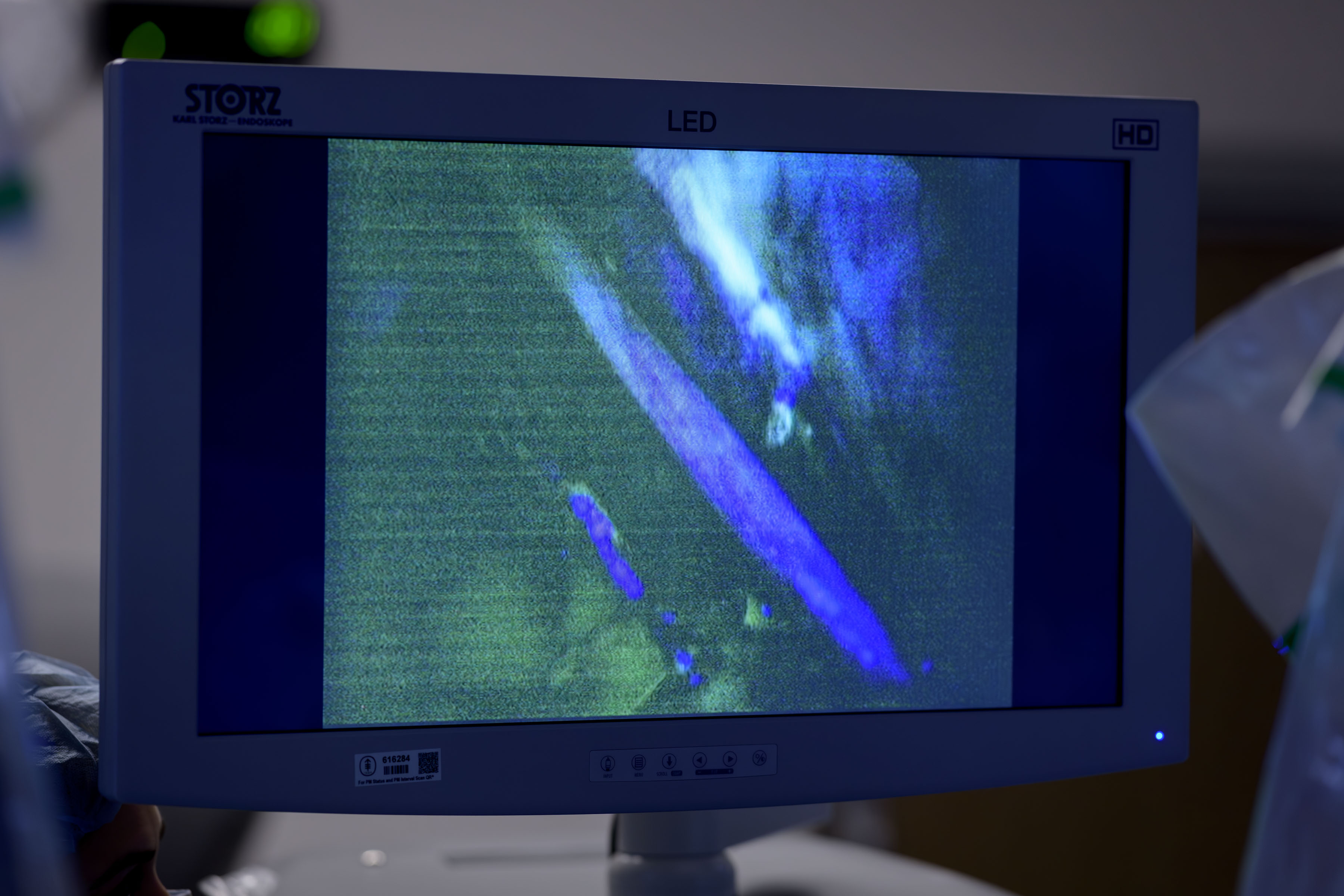
The new technology uses a fluorescent imaging agent called rizedisben (also called Illuminare-1), which binds to myelin, an insulating sheath around the nerves. When doctors shine a special blue light on the surgical area, the agent glows and increases the visibility of the nerves.
Results from a phase 1 clinical trial reported in JAMA Surgery suggest the nerve-illuminating agent is safe and effective. When given to patients undergoing robotic-assisted surgery for prostate cancer, rizedisben created lasting illumination (“sustained fluorescence”) of important nerves in the pelvis for several hours.
“This imaging approach could provide a huge benefit to men needing prostate cancer surgery,” says urologic surgeon Timothy Donahue, MD, who led the phase 1 trial. “We have urgently needed a better way to see nerves during procedures. We’re very excited about the potential of this technology, not just for prostate cancer surgery but possibly many other operations.”
An accompanying commentary in JAMA Surgery by experts at Brigham and Women’s Hospital notes that “although work remains, [Dr. Donahue and colleagues] have made clear that the future of surgery is lit.”
Rizedisben was developed through a collaboration between MSK, General Electric, and Illuminare Biotechnologies.
Nerve Imaging Tested in Prostate Cancer Surgery Clinical Trial
The standard surgery for people with prostate cancer is radical prostatectomy, a complex operation that removes the entire prostate gland along with some surrounding structures. It can be difficult for surgeons to see the nerves, especially when they are positioned close to other structures.
In the clinical trial, 38 MSK patients were scheduled for robot-assisted radical prostatectomy for newly diagnosed, localized prostate cancer. Patients received rizedisben at varying doses by intravenous infusion early in the operation.
The researchers wanted to determine the dose needed to achieve sustained fluorescence of the obturator nerve, a major nerve in the inner thigh, which can be inadvertently injured during pelvic surgeries. The doctors used this as a benchmark to see how well (and how long) rizedisben lit up the nerves.
-
In all nine patients receiving the highest dose of rizedisben, the obturator nerves brightened — usually within 30 minutes — and remained illuminated for more than three hours. (The typical radical prostatectomy takes two to three hours.)
-
Eight of the nine patients had evidence of fluorescence in the prostate neurovascular bundles — nerves located near the prostate gland that are responsible for erectile function.
- The body cleared the fluorescent agent naturally within about a day, so there were no lingering effects and no major safety events related to rizedisben.
Rizedisben Has Potential To Improve Other Kinds of Surgery
While the initial testing is in prostate cancer surgeries, Dr. Donahue and colleagues are thinking about how to expand its use to surgery for head and neck cancer, gastric cancer, colorectal cancer, breast cancer, including reconstruction, and other cancers. Future trials might also include procedures not related to cancer, such as hand and spine surgeries.

The researchers are currently designing phase 2 studies testing rizedisben for additional types of surgery.
“It takes several years for nerve function to recover after radical prostatectomy, so you have to wait a long time to see if this treatment is effective at reducing damage,” Dr. Donahue explains. “But if this agent was used for something like carpal tunnel surgery, you could get answers much sooner — like assessing hand grip strength after a month.”
Dr. Donahue believes that intraoperative imaging will transform surgery, making it both more effective and easier on patients. The pace of imaging technology is advancing rapidly.
“What was available just five years ago pales in comparison to what we can use now,” he says. “This is going to become part of how we operate in the future.”
Dr. Donahue credits MSK’s extensive team of experts in drug development for helping shepherd the drug into clinical trials.
“Illuminare-1 would never have been developed as quickly without the infrastructure at MSK,” he says. “Now that we have phase 1 study results — and it appears to be safe — we are getting a lot of interest from companies that could support further trials.”
Learn more about prostate cancer treatment at MSK.




