Separation Anxiety: Cell Division Gone Awry Leads to Genetic Chaos
Every time that a cell divides, it must distribute identical sets of DNA, packaged in chromosomes, into the daughter cells — the two new cells created. This process is usually accomplished with great precision as chromosomes separate in an orderly fashion and are parceled out to the new cells.
What is key to making this operation go smoothly are telomeres, which are stretches of DNA that cap the ends of chromosomes. Like the plastic tips on shoelaces, telomeres keep chromosome ends from fraying and sticking to one another. They are essential for maintaining the stability of genetic information as it is passed on to new cells.
Telomeres shorten a tiny bit with every cell division. Sometimes they become so short that they start fusing end to end, a development called telomere crisis, which can lead to dozens of chromosomal rearrangements in the daughter cells. It has not been clear exactly how telomere crisis causes genetic chaos, but a better understanding could provide important insights into the earliest moments when cells turn cancerous.
Now, a team of researchers at Memorial Sloan Kettering and Rockefeller University has found that proteins normally involved with immunity respond to telomere crisis in a way that promotes distinct mutational patterns in the daughter cells. The proteins appear to engage after DNA ends up in the wrong place as a result of telomere dysfunction.

“We had seen severely disordered chromosomes in cancer cells but could not explain the mechanism,” says Sloan Kettering Institute molecular biologist John Maciejowski, who led the study along with Titia de Lange of The Rockefeller University and Peter Campbell of the Wellcome Sanger Institute. “This finding clears up an important question about how aberrant chromosomal division can generate mutations found in cancer.”
The research was published on July 27 in Nature Genetics.
Custody Fight
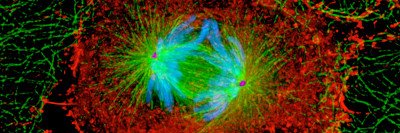
One result of telomere crisis is the generation of abnormal chromosomes. A normal chromosome has one centromere — the part of the chromosome that’s required for chromosome segregation during cell division. It helps provide a kind of handle for moving chromosomes: The segregation machinery grabs on to the handle to pull the chromosome into one of the daughter cells.
Dicentric chromosomes instead have two centromeres. Telomere fusion can generate dicentrics by joining two normal chromosomes end to end at the telomere. After this connection, one new chromosome behaves as an intact unit possessing two centromeres.
The centromeres engage in a tug-of-war as the machinery pulls at the chromosome, with each daughter cell claiming it, Dr. Maciejowski explains. The disputed chromosome can be stretched between the two daughter cells, creating a thin, temporary “bridge” of DNA between them.
Researchers noticed that chromosomes with two centromeres tend to produce mutational processes called chromothripsis and kataegis. Chromothripsis is a phenomenon in which dozens of chromosomal rearrangements occur in one or a few chromosomes. Kataegis is characterized by a large number of mutations in a small region of DNA. It was not clear exactly how dicentric chromosomes triggered these distinctive mutational patterns.
Unfamiliar Roles
The research team did a genetic experiment in which they pushed cells into telomere crisis in the absence of a protein called TREX1, which was suspected of playing a role in producing chromothripsis. In the resulting daughter cells, the rate of chromothripsis was greatly reduced. This provided evidence that TREX1 was the cause.
Dr. Maciejowski says exactly how TREX1 is connected to these abnormalities is still unknown, but the research team has a hunch.
“We think the tug-of-war over the same chromosome catapults DNA into the wrong compartment of the daughter cells,” he says. “Normally, DNA stays inside the nucleus, but telomere crisis causes some DNA to land in the cytosol, the liquid cellular compartment outside the nucleus.”
The presence of DNA in the cytosol rings alarm bells for the cells. It’s not supposed to be there, and the cells react as if they have been infected by a virus. That’s when TREX1 gets involved. TREX1 stops the immune response, turning off the alarm and clearing the DNA from the cytosol. But in resolving this problem, TREX1 — seemingly out of its league — creates another: the genetic chaos of chromothripsis.
The researchers did a similar genetic experiment to look at the possible link between another protein, APOBEC3, and kataegis. APOBEC3 normally helps disable viruses that have invaded cells, but it also appears to become active after telomere crisis. The team showed that creating telomere crisis in the absence of APOBEC3 reduced the rate of kataegis in the daughter cells, which suggests APOBEC3 is causing the kataegis.
Now that the researchers have found a mechanism connecting dicentric chromosomes to chromothripsis and kataegis, one challenge is to see how — or whether — it contributes to cancer. Although these mutational patterns are found in cancer cells, do they trigger the unchecked growth, or are they just along for the ride? And did they evolve through telomere crisis or some other undiscovered process?
“Clarifying how various proteins are involved in creating these genetic abnormalities after cell division will be critical to understanding how cancer progresses and even responds to therapy,” Dr. Maciejowski says.