The Center for Cell Engineering works in conjunction with several translational labs and core facilities within both the Sloan Kettering Institute and Memorial Hospital.
Cell Therapy and Cell Engineering Facility
Isabelle Rivière, PhD
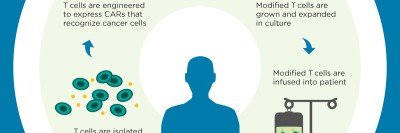
The Michael G. Harris Cell Therapy and Cell Engineering Facility (CTCEF) is a state-of-the-art resource that provides support to Memorial Sloan Kettering Cancer Center investigators who are conducting phase I and phase II clinical trials that require cell and/or vector manufacture in the realms of adoptive immunotherapies, vaccines, and stem cell-based therapies. The scope of activities includes the expansion of genetically engineered and non-engineered patient and donor cells, the manufacture of viral, bacterial, and plasmid DNA vectors, clinical cell purification, and extensive QA/QC oversight. The CTCEF was designed to enhance patient safety and to meet the current Good Manufacturing Practices requirements of the Food and Drug Administration.
Stem Cell Research Facility
Ting Zhou, PhD
The Stem Cell Research Facility expands, characterizes, and distributes non-NIH approved human embryonic stem (hES) cells isolated at the Weill-Cornell and Rockefeller University derivation core facilities.