Histiocytoses are a group of blood diseases that are diagnosed in only a few hundred people in the United States every year. Despite that rarity, researchers at Memorial Sloan Kettering have extensive experience with histiocytosis. MSK doctors care for more adults with histiocytosis than doctors at any other hospital in the country.
In recent years, MSK investigators have led a number of studies on the specific gene mutations that cause different types of histiocytoses (also called histiocytic neoplasms). On November 25, in Nature Medicine, an international team led by MSK reported findings from the largest study of its kind. They identified mutations for nearly all of the 270 people included in the study.
“We’ve known for some time that most cases of this disease are driven by a single mutation,” says MSK neurologist and histiocytosis expert Eli Diamond, one of the paper’s two senior authors. “In the past, we’ve been able to define those mutations for about 70% of patients.”
“Through the more extensive sequencing that we’ve done in this study, we can now define the mutations driving the disease in close to 100% of patients,” adds MSK physician-scientist Omar Abdel-Wahab, the paper’s other senior author. “For most of these mutations, we already have drugs to target them.”
Previous Success with Targeted Therapies
Histiocytosis occurs when the body makes an unusually large amount of abnormal white blood cells, referred to as histiocytes. These cells can build up and form tumors, which can grow in any part of the body. The bones and skin are most commonly affected.
The most common types of histiocytoses are Erdheim-Chester disease, which occurs mostly in adults; Langerhans cell histiocytosis and Rosai-Dorfman disease, which can affect both children and adults; and juvenile xanthogranuloma, which is found almost exclusively in children. All of these types were included in the study, as well as some other, rarer forms of the disease.
Thanks to earlier research done at MSK and elsewhere, experts already knew about the mutations driving many of these subtypes. That understanding has led to targeted therapies that are effective in treating them.
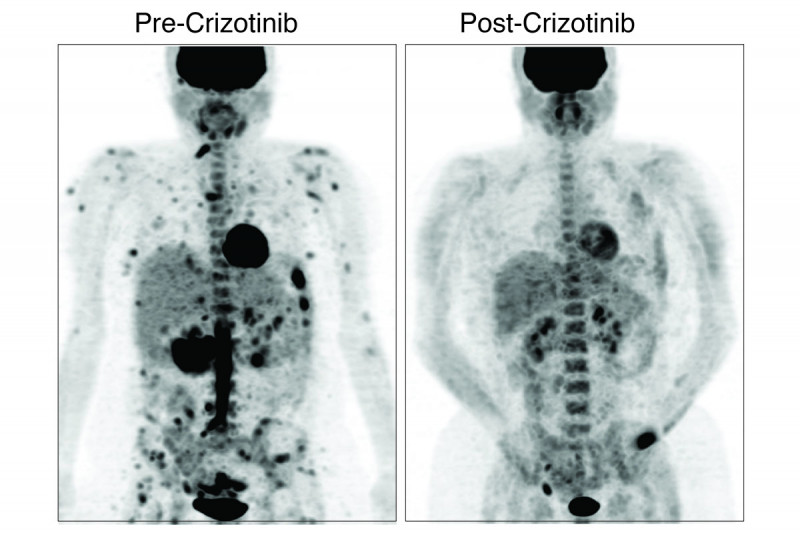
In 2017, vemurafenib (Zelboraf®) was the first drug approved for people with Erdheim-Chester disease. Vemurafenib targets mutations in a gene called BRAF. In October 2019, the US Food and Drug Administration announced that it had granted a Breakthrough Therapy Designation for the drug cobimetinib (Cotellic®) to treat histiocytosis with mutations in the genes MEK1 and MEK2. This designation indicates that the agency believes the drug is particularly promising. The clinical trials for both of these drugs were led by investigators at MSK.
“Another thing that’s important to note is that unlike treatment with most targeted therapies, where the tumors eventually become resistant to the drugs, when histiocytosis is treated with these therapies, patients’ responses tend to be long-lasting,” Dr. Diamond says. “Many people have remained on these drugs for years with durable benefits and few side effects.”
The new study opens up opportunities for even more people to be treated with targeted therapies. The researchers uncovered mutations in the RET, ALK, and NTRK genes. All of these mutations can be targeted with drugs that are already approved or are in clinical trials for other types of cancer with these mutations.
The study also reported for the first time that the gene CSF1R is implicated in certain cases of histiocytosis. CSF1R was already known to be important in the formation of a type of white blood cell called a macrophage.
“One of the strengths of this study is that it included all subtypes of histiocytosis. We have enough data to make these correlations between specific gene mutations and specific forms of histiocytosis,” says Dr. Abdel-Wahab, who leads a lab in MSK’s Human Oncology and Pathogenesis Program.
New Details about the Causes of Histiocytosis
The study revealed valuable information about the underlying origins of these diseases as well.
For example, doctors observed twins with histiocytosis. The investigators found that the common mutation driving the disease came not from the twins’ parents but from a mutation in the very early embryo that affected how their blood cells developed. These findings have implications for understanding how histiocytosis forms in many people.
Many of the patients whose data were included in the study were treated at MSK, but people treated at hospitals in Europe and other parts of the United States were included, too. Investigators from several other institutions were co-authors on the paper.
One way that the team was able to collect so many samples is through Make-an-IMPACT. This MSK initiative provides individuals with rare cancers the opportunity to receive genomic testing of their tumors at no cost. Histiocytosis is one of the cancer types included in this program.
“It’s very important that everyone who has histiocytosis gets their tumor sequenced,” Dr. Abdel-Wahab says. “It not only can help them but can also make important contributions to research.”






