By Matt Tontonoz
Maria Jasin woke up groggy from jet lag in her Paris hotel room. It was May 21, 2019. She was in the city for a scientific conference and later that morning would be heading over to the venue. But first she checked her email.
There, sitting in her inbox, was a message from the Shaw Foundation, a Hong Kong-based organization that bestows one of the most important prizes in science. She opened it and stared in disbelief.
“I thought at first they were asking me to nominate a potential prizewinner,” Dr. Jasin recalls. “It took me a moment to realize they were notifying me I was the winner.”
Known as the “Nobel of the East,” the Shaw Prize in Life Science and Medicine is given to scientists whose research has greatly benefited humanity. It comes with a cash award of $1.2 million. Dr. Jasin, a member of the Developmental Biology Program in the Sloan Kettering Institute, was being recognized for her discoveries about DNA repair. Cells use this process to fix DNA when it becomes damaged by, say, sunlight, smoking, or radiation.
Dr. Jasin began conducting her pioneering research in the 1990s. Her work eventually led to today’s cutting-edge tools for genome editing. With these methods of molecular cut and paste, scientists can make precise changes to genes, the units of heredity that specify what color your eyes are, if you’ll be tall or short, and even whether or not you have a predisposition to cancer. Genome-editing techniques offer the potential to cure people who have diseases due to inherited or acquired genetic changes.
It wasn’t a straightforward path from discovery to breakthrough — science rarely takes this route. But it was Dr. Jasin who started it all. As a recent chronicler of the genome-editing field wrote in 2018, Dr. Jasin’s findings were “the first key discovery of the Age of Editing.”
Creative Destruction
Because DNA is the blueprint for life, any damage is destined to cause trouble. The DNA in our cells is constantly being nicked and bruised by environmental toxins and the normal wear and tear of growth. If not repaired, this damage can lead to mutations: permanent changes to our genetic information.
Some kinds of DNA damage are worse than others. It’s like a car accident: You can have a fender bender, be sideswiped, or get T-boned. The worst kind of DNA damage of all — akin to a totaled car — is called a double-strand break. This is when both sides of the DNA ladder are broken, creating a gap in the double helix.
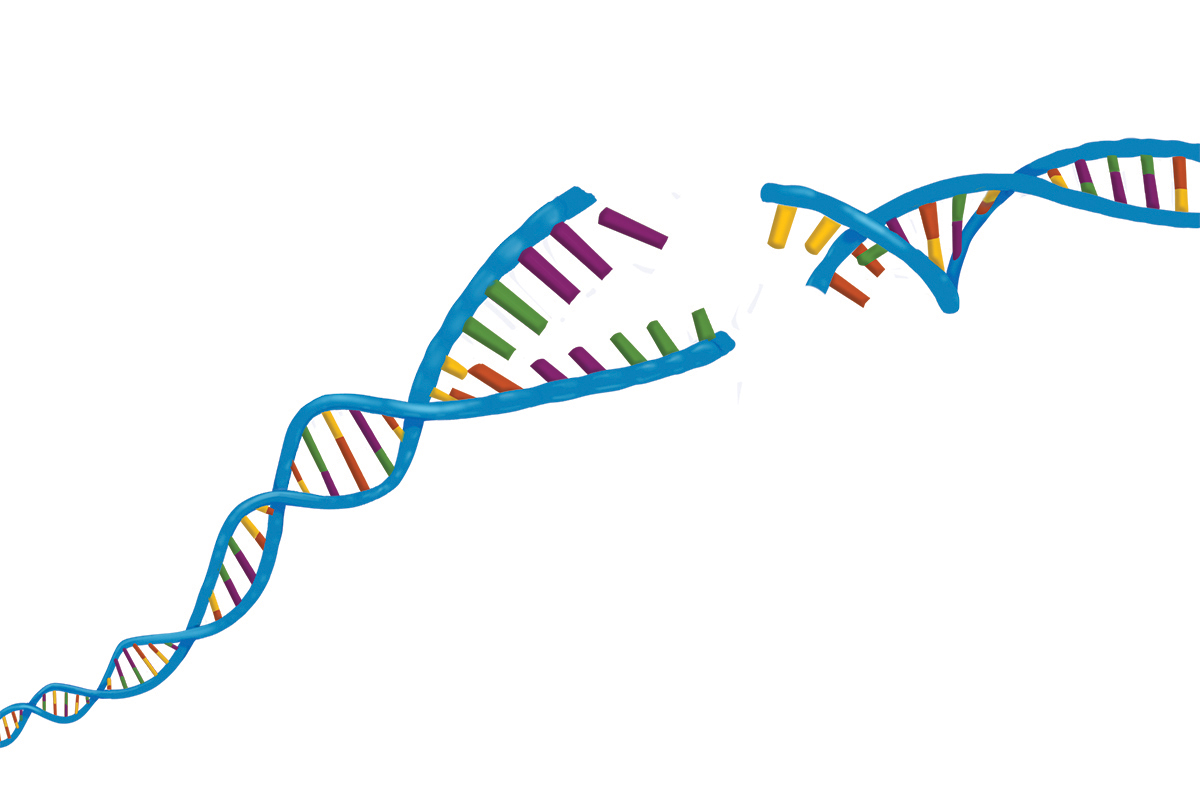
Thankfully, cells have a variety of ways to repair damaged DNA. Without this safety net, complex life would never have evolved in the first place.
Given the potential to cause harm, it may come as a surprise to learn that cells sometimes break their DNA on purpose.
“We usually think of double-strand breaks as a bad thing, as something that will put the genome at risk for mutations,” Dr. Jasin says. “But it’s actually an incredibly important part of the way we inherit traits from our parents.”
During the formation of sperm and eggs, cells break DNA strands to allow the maternal and paternal chromosomes to cross over each other and literally swap segments. This process is called meiosis. Together with SKI molecular biologist Scott Keeney, Dr. Jasin has been studying meiosis for more than 20 years. She explains that the intentional breaking and swapping is in large part why you look similar but not identical to your siblings: You each have a slightly different combination of genes from your mother and father as a result of this shuffling that occurs even before conception.
As a new member of SKI in the mid-1990s, Dr. Jasin made a startling discovery about the making and fixing of those breaks. She found a way to introduce a double-strand break at a specific spot in a mouse chromosome. The cell would repair the break but introduce mutations at the spot. But when she supplied the chromosome with an extra piece of DNA at the same time as making the break, the cell would use this new piece of DNA to complete the repair. Like a cinematographer editing a reel of film, Dr. Jasin could splice in an entirely new scene into the movie. It was the first example of what would come to be called genome editing.
Overlooked
Dr. Jasin made these pivotal observations in 1994. Yet her article “Introduction of Double-Strand Breaks into the Genome of Mouse Cells by Expression of a Rare-Cutting Endonuclease” hardly made a ripple when it first appeared in Molecular and Cellular Biology. In fact, it almost didn’t get published at all.
“I think we submitted it to four or five top-tier journals, and it got rejected from all,” Dr. Jasin recalls. “Science didn’t even send it out for review.”
Why the resistance?
“No one was interested in this type of DNA repair back then,” she recalls. “It wasn’t considered relevant to anything but sperm and egg cells where the process is highly controlled. The thought was that if it occurred in somatic [body] cells, the genome would rearrange at sequence repeats and cause disease.”
What’s more, there were other ways to modify genes at the time. These methods were somewhat crude, and they were incredibly labor-intensive, but they got the job done without involving a DNA break. As a result, although Dr. Jasin saw how her findings could take genetic engineering to the next level, few others did.
The tide began to shift in the late 1990s. That’s when scientists were becoming interested in two recently discovered cancer-related genes, BRCA1 and BRCA2. Women with mutations in either of these genes, doctors learned, were at a greatly increased risk of developing breast cancer, and they developed the disease at a much earlier age. It wasn’t yet clear why mutations in these genes caused cancer. But tantalizing new evidence would soon emerge.
Once again, Dr. Jasin’s contributions were crucial. In 1999, she and a breast oncologist working in her lab, Mary Ellen Moynahan, were the first to show that cells need a working version of the protein made from the BRCA1 gene to properly repair DNA double-strand breaks. When this protein is hobbled by a mutation, it cannot do its job. DNA damage that would normally be fixed goes unrepaired or is repaired incorrectly. Cancer is the nearly inevitable result.
For Dr. Jasin, the timing of this interest in the BRCA genes couldn’t have been better. “We were the ones with the molecular tools to actually address this question of what BRCA1 was doing in cells because of our prior work on double-strand breaks,” she says. “Here I was, one of the few people studying repair of these breaks. I’m at a cancer center. These genes are important in suppressing breast cancer, and they do so by using the type of repair that we study in the lab.”
It was quite a bit of scientific serendipity. But for Dr. Jasin, it was also deeply personal. Her own mother had died of breast cancer when Dr. Jasin was young. Finding a scientific project that might contribute to the understanding of the disease was never far from her mind.
“I remember when I was a postdoc at Stanford inquiring about what kind of research people do in breast cancer,” Dr. Jasin recalls. “At that point in time, I didn’t really see an angle for me to get into that area. But things just came together when the BRCA genes were discovered.”
Search and Replace
With the BRCA-related discoveries, scientists were finally realizing the importance of this type of DNA repair, called homologous recombination, to cancer suppression. But finding a way to apply this knowledge to genome editing was still a ways off.
One nagging feature prevented the method from gaining traction: “Our 1994 paper showed why you would want to put breaks in the genome — as a way to make edits to genes,” she says. “But we couldn’t make these breaks wherever we wanted to make them.” In other words, she could only edit one small corner of the genome, not edit any spot she wished.
Fortunately, a few researchers who had grasped the potential of Dr. Jasin’s findings were hot on this trail. They delivered the first, tentative methods for breaking DNA at specific locations around 2003.
What really caused the scientific world to stand up and take notice of Dr. Jasin’s work, however, was the discovery that bacteria had already solved this problem better than any scientist could have engineered. In 2012, researchers at the University of California, Berkeley, and the Massachusetts Institute of Technology identified a system of immune defense in bacteria called CRISPR-Cas9. This versatile enzyme complex defends bacteria against viruses by finding and chopping up specific viral DNA sequences, causing the virus to fall apart. CRISPR’s discoverers realized immediately that this bacterial find-and-cut system could be adapted into a genome-editing tool.

This was exactly the sort of targeted DNA-breaking method that Dr. Jasin and others had been dreaming of. Suddenly, with CRISPR they could make a cut at essentially any spot in the genome and then — using Dr. Jasin’s approach — swap in any piece of DNA they wanted or simply let the cell create mutations. It was a revolutionary advance.
“Maria was a true pioneer,” says Joan Massagué, Director of SKI, who recruited her to join the institute. “I watched as she faced indifference early on, and it’s gratifying now to see her being acclaimed for the singularity and importance of her findings.”
Today, thanks in part to Dr. Jasin, scientists are using genome editing to achieve amazing results. Right here at MSK, they are using these tools to engineer more-powerful immunotherapies to treat cancer, for example. Elsewhere, scientists have used CRISPR to fix the genetic mistake that leads to sickle cell anemia, an inherited condition that causes blood clots and intense pain.
Not bad for a discovery that flew under the radar for more than a decade.
“I sometimes wonder if our paper had been accepted in a more prominent journal, whether genome editing would have come sooner,” Dr. Jasin says.
Perhaps. But then again, sometimes the best science comes when no one is looking.