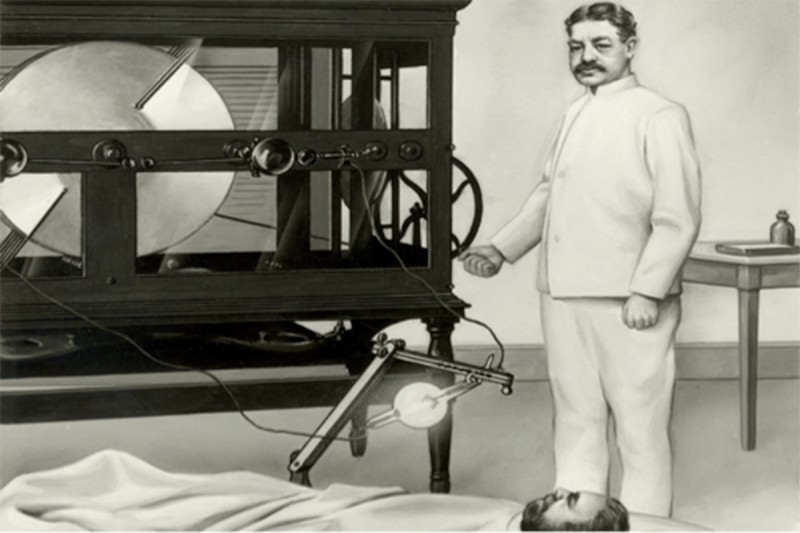
In a previous post, we recounted the history of radium therapy at Memorial Sloan Kettering. Radium, a naturally occurring radioactive element, played a central role in the evolution of MSK as a cancer hospital. But radiation therapy here was always about more than radium. A twin focus was the use of x-rays.
X-rays are waves of electromagnetic radiation — similar to radio waves and sunlight, but of much higher frequency. They are produced when electrons bombard a piece of metal. When they strike the metal, they release a packet of energy. This is an x-ray.
The German physicist Wilhelm Röntgen discovered x-rays while experimenting with electricity in his laboratory in 1895. Dr. Röntgen inadvertently took the first diagnostic x-ray when he found that his hand placed in the path of the invisible rays created a shadow of his bones on the wall.
Efforts to use x-rays therapeutically began almost immediately, once it was realized that they could target and kill cells, and that cancer cells seemed to be particularly susceptible to this form of radiation.
Today, x-ray therapy is a mainstay of cancer treatment, allowing doctors to precisely destroy tumors residing just about anywhere in the body. But there were several hurdles to overcome before x-ray therapy could become the safe and effective form of treatment it is today. Many of the most important advances were pioneered at MSK.
Precision Medicine
The story of x-ray therapy at MSK is in part a story of technological innovation — with advances in hardware came improvements in clinical care.
Memorial Hospital acquired its first two x-ray machines in 1902. These early devices had relatively low power, and the x-rays they produced deposited much of their energy in the top layers of the skin, causing burns that could sometimes be severe. Over the next few decades, the hospital acquired more powerful machines that generated higher-frequency x-rays capable of penetrating deeper into the body.
Between 1926 and 1952, MSK’s x-ray machines increased in power from 250,000 volts to 24 million volts. Today, sophisticated devices called linear accelerators (marketed under names like CyberKnife and TrueBeam) easily surpass these machines in power and precision.
Equally important as technological developments were the scientific contributions of MSK researchers, many of them associated with the Department of Medical Physics. Created in the early 1950s, the department was a unique asset among cancer hospitals. It was the first of its kind in the United States, and until this day remains one of the few freestanding medical physics departments in the country.
Having a cadre of highly skilled physicists on site meant that MSK could pioneer developments in x-ray therapy and in our understanding of the biological effects of radiation, both of which led to improved outcomes for patients.
A New Technique
Nowhere is MSK’s radiation leadership more evident than in the development of intensity-modulated radiation therapy, or IMRT, now one of the most important techniques in radiation oncology.
By the 1970s, it became clear to physicians that the doses of radiation being administered to patients were often not large enough to kill all the cancer cells in an affected area. Recurrences of cancer in the same area were all too common. But increasing the dose to try to kill more cancer cells would put patients’ overall health at risk.
In the early 1980s, a team of physicists and radiation oncologists including Clifton Ling, Zvi Fuks, and Samuel Hellman began to experiment with giving higher doses of radiation, but mapped precisely to conform to the shape of a tumor. Special hardware called multileaf collimators were added to linear accelerators and were used to sculpt the x-ray beam.
“The idea was if you could shape the field so that it matched the outline of the tumor, maybe you could increase the dose because you would not be affecting the normal tissue surrounding the tumor,” says Jean St. Germain, Vice Chair of Medical Physics at MSK.
The team started slowly, using a process called dose escalation to make sure that toxicities were manageable. The strategy of using collimators to shape the beams to match the precise contours of a tumor was called dose painting.
It was new and unknown territory.
“I can remember sitting in a room with other physicists, hearing some of the proposed dose numbers, and we were all kind of looking at each other, gasping,” Ms. St. Germain recalls. “Because we were really changing the numbers. Now, it’s not unusual to give a patient 8,000 rads or 90 centigray. We were talking then about 6,000 rads as the upper dose limits.” (A “rad” is a measure of radiation dose.)
Through their development of IMRT, Drs. Ling, Fuks, and Hellman had a lasting effect on radiation therapy, Ms. St. Germain says. “That was a triumvirate that changed completely the way in which radiation oncology was practiced.”
New Directions
MSK’s medical physicists and radiation oncologists continue to pioneer new and better ways to treat patients. One of the latest developments is radiomics — trying to understand why, given the same dose, patient A does better than patient B.
Different mutations may influence the radiosensitivity of a person’s cells. Several members of both the Medical Physics and Radiation Oncology Departments, including Chairs Joseph Deasy and Simon Powell, are tackling this question.
If a doctor knows ahead of time that a patient’s tumor will not be radiosensitive, then they can spare him the trouble and the side effects. “There’s no point in giving this patient this kind of therapy because he’s not going to benefit from it,” Ms. St. Germain says.
On the flip side, there are some types of cancer — certain breast cancers, for example — that may be more sensitive to radiation than others, because of mutations in the BRCA genes.
“Personalizing radiation treatments based on a person’s genetics — this is the next level of care,” Ms. St. Germain says.



