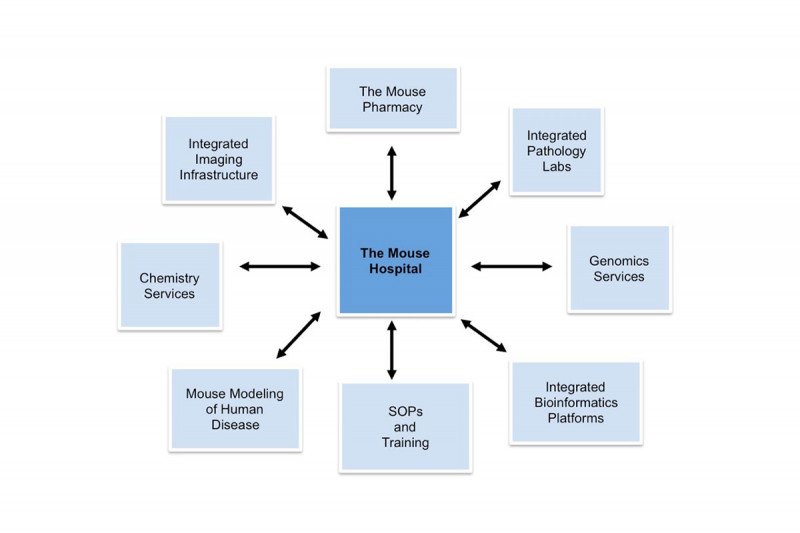
The Mouse Hospital Unit was established to strengthen infrastructural needs for the translational integration of mouse and human clinical trials at MSK. This unit ensures that mouse trials effectively mimic treatment plans of human patients, especially with regard to treatment schedule, clinical observations and outcome assessment.
Services
Toxicology and Pharmacology:
- MTD, acute and chronic toxicity studies in rodents
- PK/PD assessment of agents in mice, rats, dogs
Efficacy:
- Evaluation of efficacy of therapeutic agents in relevant mouse models
- Best route/dose/administration schedule
Technical Assistance:
- Highly specialized assistance on all aspects of in vivo studies
Consulting:
- Design of in vivo studies
- Assistance with IACUC, IRB, IND applications and grants writing
Toxicity Studies
Formulation and delivery: Compounds are formulated for in vivo delivery using a variety of commonly used vehicles, and are administered by a number of routes including: oral gavage (p.o.), intravenous (i.v.), intraperitoneal (i.p.), subcutaneous (s.c.), intratumor (i.t.) and by food and/or drinking water. Additionally, Alzet osmotic minipumps are implanted for continuous drug delivery, and slow release hormones pellets are implanted s.c.
Exploratory rodent toxicology studies: We support studies to determine Maximum Tolerated Dose (MTD) after single-dose or chronic exposure required for tolerance profiling of candidate agents.
Rodent safety toxicology studies: We perform acute single-dose or repeat-dose toxicity studies for different test articles (small, large molecules, biologics, stem cells) in mice in support of IND applications. These are performed in full compliance with Good Laboratory Practice (GLP) regulations, and can be presented in SEND compliant format. We coordinate our efforts with MSK IND/IDE Development Office, so that GLP Safety Toxicology Reports are incorporated into the Pharm/Tox section of MSK- sponsored IND applications.
PK/PD Studies
Upon compound administration (in mice, rats and dogs), plasma, organs and tumor samples are collected at multiple times to assess bioavailability and pharmacokinetic (PK), Pharmacodynamic (PD) and Toxicokinetic (TK) profile of therapeutic agents.
Staff:
Vanessa Thompson, PhD
Elisa de Stanchina, PhD
Request service:
Subject line: Toxicology service request
Send email to:
[email protected]
[email protected]
Subject line: Pharmacology services request
Send email to:
[email protected]
[email protected]
Access our websites:
Antitumor Facility
We evaluate in vivo efficacy of agents either alone or in combination with standard chemotherapy regimens against murine tumors, human tumor xenografts and transgenic mouse models of human cancer. Tumor size is monitored by caliper measurement (for s.c. xenografts) and/or in vivo imaging (for disseminated and orthotopic models).
Request service:
Send email with Subject: Efficacy Study request to:
Elisa de Stanchina
[email protected]
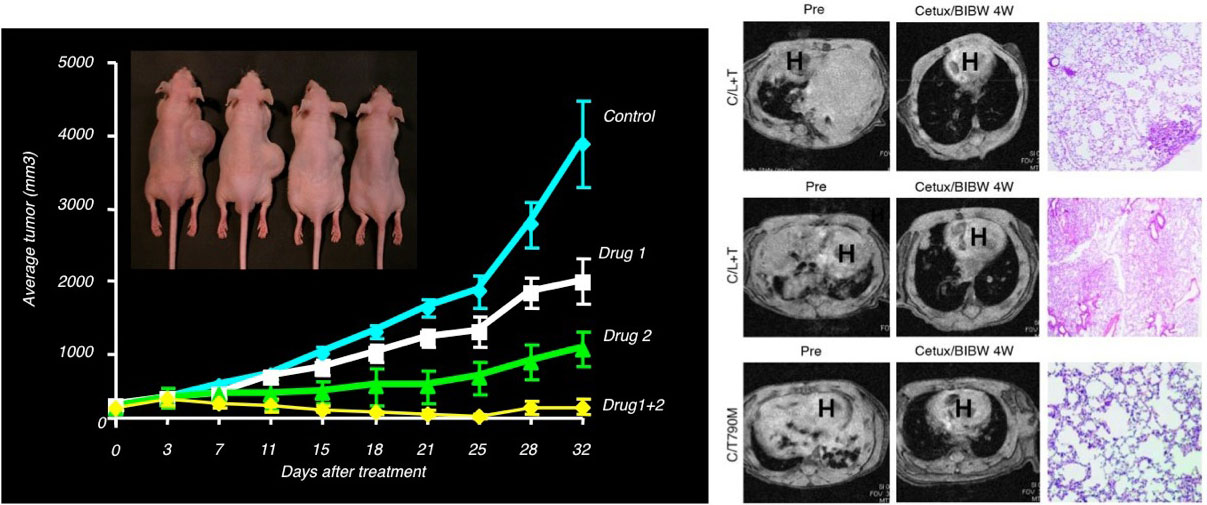
We provide technical assistance on all aspects of in vivo studies including: rodent surgeries, tumor cell implantation, tumor transplantation, bone marrow transplants, drug formulation and administration (intravenous, intraperitoneal, oral, intratumor, intradermal, subcutaneous), radiation therapy, x-ray, IVIS, MRI and PET imaging, hematology and clinical chemistry analyses, collection of fluids (blood, urine), collection and preservation of tissues/organs for either RNA/protein or HIC analysis.
- Rodent surgeries
- Blood collection
- Tissues collection and processing
- Bone marrow transplants
- Radiation therapy
- In vivo imaging
- Drug formulation and administration
List of all services provided:
| CELL LINE AND PDX ORTHOTOPIC IMPLANTATIONS |
| Bladder |
| Bone (intra femur) |
| Brain (jntracranial) |
| Breast (Mammary fat pad and intraductal) |
| Colon |
| Kidney |
| Liver |
| Lung (Intrapleural) |
| Muscle |
| Pancreas |
| Prostate |
| Ovary |
| Spleen |
| Stomach |
| Subcutaneous and intradermal |
| Thyroid |
| Tongue |
| Uterus |
| INJECTIONS and DOSING |
| Intravenous (bolus) |
| Intravenous (Infusion) |
| Intravenous (high pressure) |
| Intraperitoneum |
| Retro orbital |
| Intracardiac |
| Intramuscle |
| Sub cutaneous |
| Intratumor |
| Oral (gavage) |
| Cardiac perfusion |
| Implantation of infusion pumps via jugular catheter |
| Implantation of Alzet minipumps |
| Implantation of estrogen/testosterone pellets s.c. |
| RESECTIONS and OTHER SURGERIES |
| Sub cutaneous tumor resection |
| Mammary Fat Pad Tumor resection |
| Castration |
| Nephrectomy |
| Ovariectomy |
| Splenectomy |
| OTHER PROCEDURES |
| Blood collection (tail, cheek, retro-orbital, cardiac puncture) |
| Blood analysis (Hematology and clinical chemistry) |
| Bone Marrow Aspirates |
| Tumor/organs harvesting and processing |
| Necropsy and organ dissection services |
| Urine collection |
| Ear punching |
| Ear tagging |
| Tail Tattoo |
| Breeding (for specific projects only) |
| Cell culture (for xenograft studies) |
| Single cell suspension from Tumor samples (Gentlemacs) |
| Imaging (IVIS) – Bioluminescence and Fluorescence |
| Irradiation (whole body and X-Ray targeted radiation) |
Request service:
Send email with SUBJECT: Technical Assistance request to:
Elisa de Stanchina
[email protected]
We provide consulting services to investigators in designing and planning their preclinical studies and in analyzing and summarizing data for publications and IND applications. Additionally, we assist investigators in writing animal protocols and grant applications involving animal studies. We also typically act as a central coordinator so that studies involving support from several core facilities are carried out properly and in a timely and cost-efficient way.
- Study design (MTD, Efficacy, Safety Toxicology, PK/PD)
- Experimental data analysis
- Biostatistics support
- Writing of IRB and IACUC protocols
- Writing of IND- Enabling Toxicology studies
- Writing of SOPs
- Other aspects of IND Application (CMC and clinical protocols)
- Good Laboratory Practice (GLP) compliance
- Help with grant writing: in vivo studies design, animal number justification (VAS), IACUC, IBC and IRB compliance
Request service:
Subject line: Consultation service request
Send email to:
[email protected]