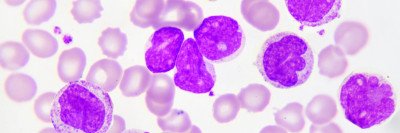
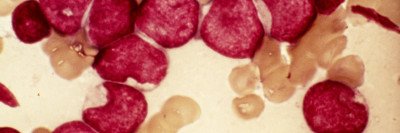
The US Food and Drug Administration has approved the drug ivosidenib (Tibsovo®) for the treatment of certain people with acute myeloid leukemia (AML) that has stopped responding to other therapies. Memorial Sloan Kettering hematologist-oncologist Eytan Stein was a co-leader of the study that led to the drug’s approval. The results of the trial were published last month in the New England Journal of Medicine (NEJM), and the drug was approved on July 20, 2018.
Ivosidenib is the first drug in a class called IDH1 inhibitors to receive FDA approval. It works in a similar way as enasidenib (Idhifa®), a drug approved in 2017 to treat AML that’s driven by a mutation in a related gene, IDH2. Both drugs represent a “new approach to treating cancer,” says Dr. Stein.
“Instead of killing cancer cells, like other leukemia drugs, it reprograms them and transforms them into normal, healthy, functioning cells,” he says.
About 10% of people with AML have mutations in the IDH1 gene, and another 15% have IDH2 mutations. These mutations are also found in other types of leukemia as well as myelodysplastic syndromes, glioblastoma, and bile duct cancer. Targeting these mutations is a growing area of cancer drug development.
MSK President and CEO Craig Thompson led the basic science research that explains how IDH1 mutations drive AML, in collaboration with MSK physician-scientists Ross Levine and Omar Abdel-Wahab. The Peter and Susan Solomon Family Foundation supported that research, which was first reported in 2010. The investigators found that the mutations produce a cancer-causing enzyme called hydroxyglutarate (2HG). This enzyme stops the development of the blood cells called myeloid cells when they are in an immature form, which leads to leukemia.
Ivosidenib brings down the level of 2HG, so the blood cells can begin to develop normally again.
The NEJM study was a multicenter phase I trial that reported data on 125 people whose cancer had stopped responding to other treatments. The researchers found that of those treated with ivosidenib, almost 42% responded. Nearly 22% had a complete remission, meaning that their cancer was no longer detectable. The overall survival was longer than what would be expected for people with this stage of AML, and severe side effects were rare.
MSK Leukemia Service Chief Martin Tallman also participated in the study.
Ivosidenib and enasidenib are both made by Agios Pharmaceuticals.