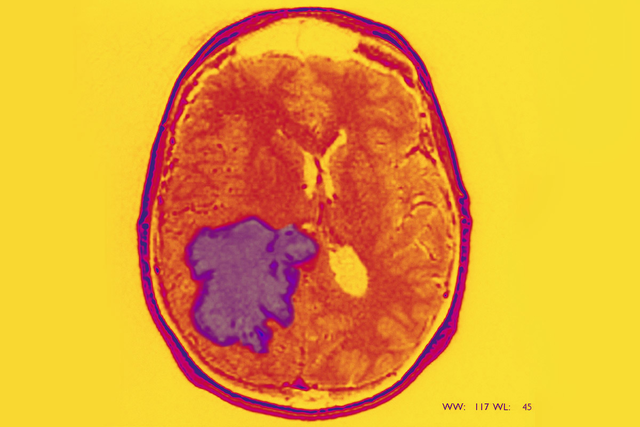
The type of brain tumor known as glioblastoma (GBM) is one of the most difficult cancers to treat. Complete removal by surgery is impossible because of where and how these tumors infiltrate brain tissue. Additionally, the most commonly used treatments for glioblastoma — radiation therapy and the chemotherapy drug temozolomide (Temodar®) — are not very effective over the long term.
Researchers in Memorial Sloan Kettering’s Brain Tumor Center, led by developmental biologist Luis F. Parada, are focused on finding more effective ways of attacking this deadly cancer. In a study published in Nature, they report on the identification of a compound that kills glioblastoma cells using a mechanism that’s completely different from earlier treatments. The scientists say one of the keys to finding better drugs is developing models that accurately reflect the cells that make up these tumors.
“We use genetically engineered mouse models to study the biological features of glioblastoma in a way that you cannot do with other laboratory methods,” Dr. Parada says. “We’ve found that a critical component of cells within these tumors are cancer stem cells. This required us to take a different approach to developing new therapies.”
Focusing on Cancer Stem Cells
Like normal stem cells, cancer stem cells can give rise to many other types of cells. This means they have the ability to rebuild a tumor, even after most of it has been removed, leading to cancer relapse and metastasis.
“The pharmaceutical industry has traditionally used established cancer cell lines to screen for new drugs, but these cell lines don’t always reflect how cancer behaves in the body,” explains Dr. Parada, whose lab is in the Sloan Kettering Institute’s Cancer Biology and Genetics Program. Cell lines are idiosyncratic populations derived from a single cancer cell that are grown in the lab.
“The therapies that are currently in use were designed to target cells that are rapidly dividing,” he adds. “But what we’ve concluded in our studies is that glioblastoma stem cells divide relatively slowly within tumors, leaving them unaffected by these treatments.” Even if most of the tumor is destroyed, the stem cells allow it to regrow.
Glioblastoma is driven primarily by a small number of gene mutations, including three genes that have been used to model the tumor in mice. The investigators in Dr. Parada’s lab demonstrated that when mice were engineered to carry these three mutations, 100% of them developed glioblastoma.
Next they pooled the cells from the mouse-derived tumors. Their hope was that this population of cells would be more accurate than traditional cell lines in reflecting the range of cell types making up a typical glioblastoma tumor. With these cells in hand, the team was ready to use them to begin looking for new drugs.
Finding a Needle in a Haystack of Drugs
What they sought were compounds that could kill glioblastoma tumor cells, including cancer stem cells, without killing normally dividing cells.
Using high-throughput screening, a method in which hundreds of thousands of compounds can be evaluated in a single test, the list of potential drugs was whittled from 200,000 to 5,000 to 2,000 to 60. More in-depth studies were conducted on the 60 most promising compounds.
One drug — which the team eventually dubbed Gboxin, short for “glioblastoma toxin” — rose to the top. It was effective at curbing the growth of glioblastoma tumors in mice and did not seem to make them sick.
“Once we identified this compound, the next step was to test it on glioblastoma cells derived from patients with glioblastoma,” Dr. Parada says. “We found that the human glioblastoma cells all succumbed to Gboxin.”
After the researchers showed that Gboxin was effective at treating glioblastoma in mice and killing human glioblastoma cells, they set out to determine how and why it worked.
An Unexpected Mechanism Opens Up New Possibilities for Treatment
Through a series of complex experiments, they made an unexpected discovery: that Gboxin killed cancer stem cells by affecting a specific component of their metabolism and starving them of energy. Several labs at MSK study the metabolism of cancer cells, including those of MSK President and CEO Craig Thompson and Sloan Kettering Institute cell biologist Lydia Finley. But until now, this hasn’t been a focus of Dr. Parada’s work.
Specifically, the researchers found that Gboxin prevents cells from making ATP — the cell’s energy currency — by way of a process called oxidative phosphorylation. This highly efficient process of metabolism takes place in structures called mitochondria, sometimes referred to as a cell’s energy factories.
“Stem cells are very specialized and need a lot of energy to do their thing,” Dr. Parada says. “When Gboxin accumulates within cancer stem cells, it essentially strangles the mitochondria and shuts energy production down.”
Other dividing cells in the body, including other cancer cells, rely less on oxidative phosphorylation than stem cells. They employ instead a type of metabolism called glycolysis, which is better at generating the building materials required to make new cells. This makes them much less sensitive to Gboxin.
“Ideally, what we want in a glioblastoma drug is something that targets the cancer stem cells specifically, while sparing normal cells.” Dr. Parada says. “That’s what Gboxin seems to do.”
A Promising Start to Research
There is still a lot of work to be done before Gboxin can be tested in clinical trials in humans. Among the next steps, the investigators need to determine that Gboxin will be able to cross what is called the blood-brain barrier. If it can’t, a version that is able to do so will need to be developed. Much more research is needed on the potential side effects of the drug as well.
Dr. Parada explains that because many tumors develop from cancer stem cells, the findings about Gboxin and glioblastoma stem cells may be applicable to other types of cancer. “What we know about cancer stem cells in general suggests that they all use similar mechanisms for their growth and proliferation,” he concludes. “If this drug turns out be effective against glioblastoma, we’ll be very happy. If it turns out to work against other types of cancer, that would be great, too.”